Hydrazonoyl Chlorides as Precursors for Synthesis of Novel Bis-Pyrrole Derivatives
Abstract
1. Introduction
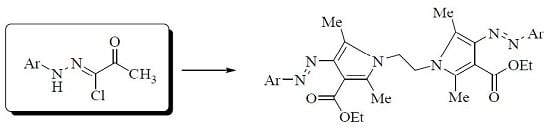
2. Results and Discussion
2.1. Chemistry
2.2. Antimicrobial Evaluation
2.3. Docking and Molecular Modeling
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Synthesis of Bis-Pyrrole Derivatives 5a–e
3.2. Agar Diffusion Well Method to Determine the Antimicrobial Activity
3.3. Docking Studies
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mabkhot, Y.N.; Barakat, A.; Al-Majid, A.M.; Alshahrani, S.; Yousuf, S.; Choudhary, M.I. Synthesis, reactions and biological activity of some new bis-heterocyclic ring compounds containing sulphur atom. Chem. Cent. J. 2013, 7, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Panwar, H. Synthesis and pharmacological evaluation: antimicrobial, anti-inflammatory, analgesic, ulcerogenic properties of several bis-heterocyclic derivatives. Indonesian J. Pharm. 2015, 26. [Google Scholar] [CrossRef]
- Bhanu Prakash, T.; Dinneswara Reddy, G.; Padmaja, A.; Padmavathi, V. Synthesis and antimicrobial activity of amine linked bis- and tris-heterocycles. Eur. J. Med. Chem. 2014, 82, 347–354. [Google Scholar]
- Tripathi, A.; Fornabaio, M.; Kellogg, G.E.; Gupton, J.T.; Gewirtz, D.A.; Yeudall, W.A.; Vega, N.E.; Mooberry, S.L. Docking and hydropathic scoring of polysubstituted pyrrole compounds with antitubulin activity. Bioorg. Med. Chem. 2008, 16, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Micheli, F.; di Fabio, R.; Cavanni, P.; Rimland, J.M.; Capelli, A.M.; Chiamulera, C.; Corsi, M.; Corti, C.; Donati, D.; Feriani, A.; et al. Synthesis and pharmacological characterisation of 2,4-dicarboxy-pyrroles as selective non-competitive mGluR1 antagonists. Bioorg. Med. Chem. 2003, 11, 171–183. [Google Scholar] [CrossRef]
- Krutzik, P.O.; Chamberlin, A.R. Rapid solid-phase synthesis of DNA-binding pyrrole-imidazole polyamides. Bioorg. Med. Chem. Lett. 2002, 12, 2129–2132. [Google Scholar] [CrossRef]
- Lv, K.; Wang, L.L.; Liu, M.L.; Zhou, X.B.; Fan, S.Y.; Liu, H.Y.; Zheng, Z.B.; Li, S. Synthesis and antitumor activity of 5-[1-(3-(dimethylamino)propyl)-5-halogenated-2-oxoindolin-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxamides. Bioorg. Med. Chem. Lett. 2011, 21, 3062–3065. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Romagnoli, R.; Pavani, M.G.; Nunez, M.D.C.; Bingham, J.P.; Hartley, J.A. Benzoyl and cinnamoyl nitrogen mustard derivatives of benzoheterocyclic analogues of the tallimustine: Synthesis and antitumour activity. Bioorg. Med. Chem. 2002, 10, 1611–1618. [Google Scholar] [CrossRef]
- Kang, S.Y.; Park, E.J.; Park, W.K.; Kim, H.J.; Jeong, D.; Jung, M.E.; Song, K.S.; Lee, S.H.; Seo, H.J.; Kim, M.J.; et al. Arylpiperazine-containing pyrrole 3-carboxamide derivatives targeting serotonin 5-HT2A, 5-HT2C and the serotonin transporter as a potential antidepressant. Bioorg. Med. Chem. Lett. 2010, 20, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.M.; O’Toole-Colin, K.; Getzel, A.; Argenti, A.; Evans, M.A.; Smith, D.C.; Dalglish, G.A.; Rifat, S.; Wilson, D.L.; Taylor, B.M.; et al. Lipid-lowering effects of ethyl 2-phenacyl-3-aryl-1H-pyrrole-4-carboxylates in rodents. Molecules 2004, 9, 134–157. [Google Scholar] [CrossRef] [PubMed]
- Almerico, A.M.; Diana, P.; Barraja, P.; Dattolo, G.; Mingoia, F.; Putzolu, M.; Perra, G.; Milia, C.; Musiu, V.; Marongiu, M.E. Glycosidopyrroles. Part 2. Acyclic derivatives: 1-(1,3-dihydroxy-2-propoxy)methyl pyrroles as potential antiviral agents. Farmaco 1997, 52, 667–672. [Google Scholar] [PubMed]
- Ramazanzadeh, R.; Nasiri, F. Dimethyl 2-hydroxy-1-methyl-3-[2-Oxo-2-Phenylethylidene]-2-Phenyl-1,2-Dihydro-3H-Pyrrole-4,5-dicarboxylate: A potential lead compound as anti-gram-positive and anti-gram-negative agent. J. Applied Sci. 2009, 9, 2198–2200. [Google Scholar] [CrossRef]
- Shawali, A.S.; Abdallah, M.A. The Chemistry of Heterocyclic Hydrazonoyl Halides. Adv Heterocycl. Chem. 2001, 80, 277–338. [Google Scholar]
- Shawali, A.S.; Edrees, M.M. Reactions of nitrilimines with heterocyclic amines and enamines. Convenient methodology for synthesis and annulation of Heterocycles. Arkivoc 2006, 9, 292–365. [Google Scholar] [CrossRef]
- Kheder, N.A.; Mabkhoot, Y.N. Synthesis and antimicrobial studies of some novel bis-[1,3,4]thiadiazole and bis-thiazole pendant to thieno[2,3-b]thiophene moiety. Int. J. Mol. Sci. 2012, 13, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Kheder, N.A.; Al-Majid, A.M. Facile and convenient synthesis of new thieno[2,3-b]thiophene derivatives. Molecules 2010, 15, 9418–9426. [Google Scholar] [CrossRef] [PubMed]
- Darwish, E.S.; Kheder, N.A.; Farag, A.M. Synthesis and antimicrobial evaluation of some new pyridine based Heterocycles. Heterocycles 2010, 81, 2247–2256. [Google Scholar]
- Mabkhot, Y.N.; Kheder, N.A.; Farag, A.M. An efficient synthesis of new thiazole based heterocycles. Heterocycles 2010, 81, 2369–2376. [Google Scholar]
- Dawood, K.M.; Kheder, N.A.; Ragab, E.A.; Mohamed, S.N. A facile access to some new pyrazole, 1,3,4-thiadiazole, and thiophene derivatives via B-ketosulfones. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 330–339. [Google Scholar] [CrossRef]
- Farag, A.M.; Kheder, N.A.; Mabkhot, Y.N. Synthesis and antimicrobial evaluation of new pyrazole, thiophene, thiazole and 1,3,4-thiadiazole derivatives incorporating pyrimidine ring. Heterocycles 2009, 78, 1787–1798. [Google Scholar] [CrossRef]
- Kheder, N.A. Convenient synthesis of novel bis(hydrazone) and bis(indole) derivatives. Heterocycles 2009, 78, 1281–1288. [Google Scholar] [CrossRef]
- Kheder, N.A. Synthesis of some novel bis(pyrazole), bis(pyridine) and bis-pyrazolo[5,1-c]-1,2,4-triazine derivatives. Heterocycles 2009, 78, 1815–1822. [Google Scholar] [CrossRef]
- Kheder, N.A.; Mabkhoot, Y.N.; Farag, A.M. Synthesis and antimicrobial evaluation of some bis(thioxopyridine), bis(pyrazolo[3,4-b]pyridine), bis(thieno[2,3-b]pyridine), bis(1,3,4-thiadiazole) and bis(thiophene) derivatives. Heterocycles 2008, 75, 2937–2948. [Google Scholar]
- Kheder, N.A.; Mabkhot, Y.N.; Farag, A.M. Facile and convenient synthesis of pyrazole, pyridine, pyridazine, pyrazolo[3,4-b]pyridine, and pyrazolo[5,1-c][1,2,4]-triazine derivatives. Synth. Commun. 2008, 38, 3170–3182. [Google Scholar] [CrossRef]
- Kheder, N.A.; Farghaly, T.A. Bis-Hydrazonoyl chloride as precursors for synthesis of novel polysubstituted bis-azoles. Arab. J. Chem. 2013. [Google Scholar] [CrossRef]
- Shyadligeri, A.S.; Gadaginamath, G.S.; Subramanian, L.R. One-pot synthesis of a novel 1,1′-bridged bis(benzo(g)indole) diester and the preparation of some of its derivatives. J. Chem. Res. 1996, 2, 114–115. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Jin, S.; Shang, Z.; Huang, S.; Liu, B.; Guo, J. Diethyl (Z,Z)-3,3′-(ethane-1,2-diyldi-imino)dibut-2-enoate. Acta Crystallogr. C 2004, 60, o176–o177. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, W.; Platz, L. Ueber eine neue Bildungsweise von Osotetrazonen. Ber. Dtsch. Chem. Ges. 1905, 38, 2986–2990. [Google Scholar] [CrossRef]
- Abushamleh, A.S.; Al-Aqarbeh, M.M.; Day, V. Transition Metal Complexes of Derivatized Chiral Dihydro-1,2,4-triazin-6-ones. Template Synthesis of Nickel(II) Tetraaza-(4N-M) Complexes Incorporating the Triazinone Moiety. Am. J. Appl. Sci. 2008, 5, 750–754. [Google Scholar] [CrossRef]
- Eweiss, N.F.; Abdelhamid, A.O. Synthesis of Heterocycles. Part II. New routes to acetyl thiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Tanoli, S.A.K.; Tanoli, N.U.; Usmani, S.; Ferreira, A.G. The exploration of interaction studies of smaller size, mostly ignored yet intrinsically inestimable molecules towards BSA; An example of STD and DOSY NMR. Cent. Eur. J. Chem. 2014, 12, 332–340. [Google Scholar] [CrossRef]
- Protein Data Bank. Available online: http://www.rcsb.org/pdb (accessed on 1 March 2016).
- Smania, A.; Monache, F.D.; Smania, E.F.A.; Cuneo, R.S. Triterpenes and sterols from ganoderma australe (Fr) Pat. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 1999, 1, 325–334. [Google Scholar]
- Sample Availability: Samples of the compounds 5a–e are available from the author.







| Microorganisms | Compound Tested | Standard (30 µg/mL) | |||
|---|---|---|---|---|---|
| 5a | 5b | 5d | 5e | ||
| Fungi | Itraconazole | ||||
| Aspergillus fumigatus (RCMB 002003) | 18.2 ± 0.84 | 11.3 ± 0.68 | 21.1 ± 0.2 | 16.3 ± 0.09 | 22 ± 0.1 |
| Geotrichumcandidum (RCMB 052006) | 18.9 ±0.35 | 12.6 ±0.54 | 19.3 ± 0.05 | 15.4 ± 0.1 | 26 ± 0.3 |
| Syncephalastrum racemosum (RCMB 005003) | 11.2 ± 0.44 | NA | 16.4 ± 0.08 | 14.2 ± 0.05 | 19 ± 0.1 |
| Candida albicans (RCMB 005002) | NA | NA | NA | NA | 24 ± 0.1 |
| Gram-positive Bacteria | Penicillin G | ||||
| Staphylococcus aureus (RCMB 000106) (MSSA) | 17.8 ± 0.58 | 10.7 ± 0.36 | 21.4 ± .03 | 17.9 ± 0.03 | 27.4 ± 0.08 |
| Bacillis subtilis (RCMB 000107) | 17.9 ± 0.46 | 11.1 ± 0.72 | 26.1 ± 0.04 | 15.2 ± 0.04 | 28.6 ± 0.03 |
| Gram-negative Bacteria | Streptomycin | ||||
| Pseudomonas aeruginosa (RCMB 000102) | NA | NA | 19.9 ± 0.09 | NA | 26.3 ± 0.03 |
| Escherichia coli (RCMB 000103) | 12.6 ± 0.57 | 12.4 ±0.04 | 17.2 ± 0.2 | 13.4 ± 0.04 | 30.1 ± 0.07 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheder, N.A. Hydrazonoyl Chlorides as Precursors for Synthesis of Novel Bis-Pyrrole Derivatives. Molecules 2016, 21, 326. https://doi.org/10.3390/molecules21030326
Kheder NA. Hydrazonoyl Chlorides as Precursors for Synthesis of Novel Bis-Pyrrole Derivatives. Molecules. 2016; 21(3):326. https://doi.org/10.3390/molecules21030326
Chicago/Turabian StyleKheder, Nabila Abdelshafy. 2016. "Hydrazonoyl Chlorides as Precursors for Synthesis of Novel Bis-Pyrrole Derivatives" Molecules 21, no. 3: 326. https://doi.org/10.3390/molecules21030326
APA StyleKheder, N. A. (2016). Hydrazonoyl Chlorides as Precursors for Synthesis of Novel Bis-Pyrrole Derivatives. Molecules, 21(3), 326. https://doi.org/10.3390/molecules21030326