sym-Trisubstituted 1,3,5-Triazine Derivatives as Promising Organic Corrosion Inhibitors for Steel in Acidic Solution
Abstract
:1. Introduction
2. Results and Discussion
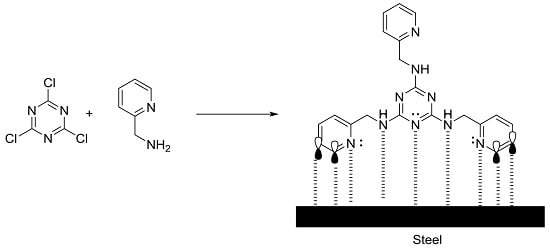
2.1. Synthesis of sym-Trisubstituted-s-triazines 4, 6, and 8
2.2. Potentiodynamic Polarization Measurements
2.3. EIS Studies
2.4. Adsorption Isotherm
3. Experimental Section
3.1. General Information
3.2. General Method for the Synthesis of 1,3,5-Triazine Derivatives
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pereira, S.S.A.A.; Pegas, M.M.; Fernandez, T.L.; Magalhães, M.; Schontag, T.G.; Lago, D.C.; de Senna, L.F.; D’Elia, E. Inhibitory action of aqueous garlic peels extract on the corrosion of carbon steel in HCl solution. Corros. Sci. 2012, 65, 360–366. [Google Scholar]
- Satapathy, A.K.; Gunasekaran, G.; Sahoo, S.C.; Amit, K.; Rodrigues, P.V. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 2009, 51, 2848–2856. [Google Scholar] [CrossRef]
- Da Rocha, J.C.; Gomes, J.A.C.P.; D’Elia, E. Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peels aqueous extracts. Corros. Sci. 2010, 52, 2341–2348. [Google Scholar] [CrossRef]
- Tebbji, K.; Oudda, H.; Hammouti, B.; Benkaddour, M.; El kodadi, M.; Malek, F.; Ramdani, A. Inhibitive action of two bipyrazolic isomers towards corrosion of steel in 1 M HCl solution. Appl. Surf. Sci. 2005, 241, 326–334. [Google Scholar] [CrossRef]
- Lagrenée, M.; Mernari, B.; Chaibi, N.; Traisnel, M.; Vezin, H.; Bentiss, F. Investigation of the inhibitive effect of substituted oxadiazoles on the corrosion of mild steel in HCl medium. Corros. Sci. 2001, 43, 951–962. [Google Scholar]
- Yang, Y.Z.; Li, G.J.; Zhou, F.D.; Chen, X.J. The effect of sudden change in pipe diameter on flow patterns of air-water two-phase flow in vertical pipe (II) sudden-expansion cross-section. Chin. J. Chem. Eng. 2001, 9, 221–223. [Google Scholar]
- Bouklah, M.; Attayibat, A.; Kertit, S.; Ramdani, A.; Hammouti, B. A pyrazine derivative as corrosion inhibitor for steel in sulphuric acid solution. Appl. Surf. Sci. 2005, 242, 399–406. [Google Scholar] [CrossRef]
- Xiao, Y.M.T.; Huang, Y.; Meng, C.; Guo, Y.H. Kinetics of asymmetric reduction of phenylglyoxylic acid to R-(−)-mandelic acid by Saccharomyces cerevisiae FD11b. Chin. J. Chem. Eng. 2006, 14, 73–80. [Google Scholar] [CrossRef]
- Gan, Y.P.; Zhang, W.K.; Huang, H.; Xia, X.H.; Cheng, Y.S. Industrial synthesis of N-methylhydroxylamine hydrochloride by electrochemical reduction of nitromethane. Chin. J. Chem. Eng. 2006, 14, 649–653. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Chaibi, N.; Mernari, B.; Vezin, H.; Lagrenée, M. 5-bis(n-methoxyphenyl)-1,3,4-oxadiazoles used as corrosion inhibitors in acidic media: Correlation between inhibition efficiency and chemical structure. Corros. Sci. 2002, 44, 2271–2285. [Google Scholar] [CrossRef]
- El-Rehim, S.S.A.; Refaey, S.A.M.; Taha, F.; Saleh, M.B.; Ahmed, R.A. Corrosion inhibition of mild steel in acidic medium using 2-amino thiophenoland 2-cyanomethyl benzothiazole. J. Appl. Electrochem. 2001, 31, 429–435. [Google Scholar] [CrossRef]
- Chetouani, A.; Aouniti, A.; Hammouti, B.; Benchat, N.; Benhadda, T.; Kertit, S. Corrosion inhibitors for iron in hydrochloride acid solution by newly synthesized pyridazine derivatives. Corros. Sci. 2003, 45, 1675–1684. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Lagrenée, M. The substituted 1,3,4-oxadiazoles: A new class of corrosion inhibitors of mild steel in acidic media. Corros. Sci. 2000, 42, 127–146. [Google Scholar] [CrossRef]
- Xu, C.M.; Zhang, Y.H.; Cheng, G.X.; Zhu, W.S. Corrosion and electrochemical behavior of 316L stainless steel in sulfate-reducing and iron-oxidizing bacteria solutions. Chin. J. Chem. Eng. 2006, 14, 829–834. [Google Scholar]
- Cao, C. On electrochemical techniques for interface inhibitor research. Corros. Sci. 1996, 38, 2073–2082. [Google Scholar] [CrossRef]
- Mernari, B.; Elattari, H.; Traisnel, M.; Bentiss, F.; Lagrenee, M. Inhibiting effects of 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles on the corrosion for mild steel in 1 M HCl medium. Corros. Sci. 1998, 40, 391–399. [Google Scholar] [CrossRef]
- Elachouri, M.; Infante, M.R.; Izquierdo, F.; Kertit, S.; Gouttaya, H.M.; Nciri, B. Synthesis of some cationic gemini surfactants and their inhibitive effect on iron corrosion in hydrochloric acid medium. Corros. Sci. 2001, 43, 19–35. [Google Scholar] [CrossRef]
- Gao, Z.M.; Shi, L.T. Effect of temperature on gas hold-up in aerated stirred tanks. Chin. J. Chem. Eng. 2003, 11, 204–207. [Google Scholar]
- Hammouti, B.; Salghi, R.; Kertit, S. Electrochemical behavior of lead in 0.3M HCl in presence of pyrazolic compounds. J. Electrochem. Soc. 1998, 47, 31–34. [Google Scholar]
- Abdallaha, M.S.; Al Karaneea, O.; Abdel Fataha, A.A. Inhibition of acidic and pitting corrosion of Nickel using natural black Cumin oil. Chem. Eng. Commun. 2010, 197, 1446–1454. [Google Scholar] [CrossRef]
- Okafor, P.C.; Zheng, Y.G. Synergistic inhibition behavior of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros. Sci. 2009, 51, 850–859. [Google Scholar] [CrossRef]
- Okafor, P.C.; Liu, X.; Zheng, Y.G. Corrosion inhibition of mild steel by ethylamino imidazoline derivative in CO2-saturated solution. Corros. Sci. 2009, 51, 761–768. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Li, Y.; Li, S.; Wang, L. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]
- Mahdavian, M.; Attar, M.M. Electrochemical behavior of some transition metal acetylacetonate complexes as corrosion inhibitors for mild steel. Corros. Sci. 2009, 51, 409–414. [Google Scholar] [CrossRef]
- Liu, X.; Okafor, P.C.; Zheng, Y.G. The inhibition of CO2 corrosion of N80 mild steel in single liquid phase and liquid/particle two-phase flow by aminoethyl imidazoline derivatives. Corros. Sci. 2009, 51, 744–751. [Google Scholar] [CrossRef]
- Liu, F.G.; Du, M.; Zhang, J.; Qiu, M. Electrochemical behavior of Q235 steel in saltwater saturated with carbon dioxide based on new imidazoline derivative inhibitor. Corros. Sci. 2009, 51, 102–109. [Google Scholar] [CrossRef]
- Riggs, O.L. Corrosion Inhibitors, 2nd ed.; Nathan, C.C., Ed.; NACE: Houston, TX, USA, 1973. [Google Scholar]
- Negam, N.A.; Kandile, N.G.; Aiad, I.A.; Mohammad, M.A. New eco-friendly cationic surfactants: Synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1N HCl. Colloids Surf. A Physicochem. Eng. Asp. 2011, 391, 224–233. [Google Scholar] [CrossRef]
- Selvi, S.T.; Raman, V.; Rajendran, N. Corrosion inhibition of mild steel by benzotriazole derivatives in acidic medium. J. Appl. Electrochem. 2003, 33, 1175–1182. [Google Scholar] [CrossRef]
- Tang, L.B.; Mu, G.N.; Liu, G.H. The effect of neutral red on the corrosion inhibition of cold rolled steel in 1.0 M hydrochloric acid. Corros. Sci. 2003, 45, 2251–2262. [Google Scholar] [CrossRef]
- Asan, A.; Soylu, S.; Kıyakb, T.; Yıldırımb, F.; Öztaşc, S.G.; Ancınc, N.; Kabasakaloğlu, M. Investigation on some Schiff bases as corrosion inhibitors for mild steel. Corros. Sci. 2006, 48, 3933–3944. [Google Scholar] [CrossRef]
- El Mehdi, B.; Mernari, B.; Traisnel, M.; Bentiss, F.; Lagrenee, M. Synthesis and comparative study of the inhibitive effect of some new triazole derivatives towards corrosion of mild steel in hydrochloric acid solution. Mater. Chem. Phys. 2003, 77, 489–496. [Google Scholar] [CrossRef]
- Wang, H.L.; Fan, H.B.; Zheng, J.S. Corrosion inhibition of mild steel in hydrochloric acid solution by a mercapto-triazole compound. Mater. Chem. Phys. 2003, 77, 655–661. [Google Scholar] [CrossRef]
- Shukla, S.K.; Ebenso, E.E. Corrosion inhibition, adsorption behavior and thermodynamic properties of streptomycin on mild steel in hydrochloric acid medium. Int. J. Electrochem. Sci. 2011, 6, 3277–3291. [Google Scholar]
- De Souza, F.S.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Lece, H.D.; Emregul, K.C.; Atakol, O. Difference in the inhibitive effect of some Schiff base compounds containing oxygen, nitrogen and sulfur donors. Corros. Sci. 2008, 50, 1460–1474. [Google Scholar] [CrossRef]
- Heakal, F.E.; Fouda, A.S.; Radwan, M.S. Some new thiadiazole derivatives as corrosion inhibitors for 1018 carbon steel dissolution in sodium chloride solution. Int. J. Electrochem. Sci. 2011, 6, 3140–3163. [Google Scholar]
- Heakal, F.E.; Fouda, A.S.; Radwan, M.S. Inhibitive effect of some thiadiazole derivatives on C-steel corrosion in neutral sodium chloride solution. Mater. Chem. Phys. 2011, 125, 26–36. [Google Scholar] [CrossRef]
- EL-Mahdy, G.A.; Atta, A.M.; Al-Lohedan, H.A.; Ezzat, A.O. Synthesis of water soluble hyperbrached poly(amino-ester) as corrosion inhibitors for steel. Int. J. Electrochem. Sci. 2014, 4, 7925–7934. [Google Scholar]
- Mahapatra, S.S.; Yadav, S.K.; Yoo, H.J.; Whan, J.; Park, C.J.-S. Highly branched polyurethane: Synthesis, characterization and effects of branching on dispersion of carbon nanotubes. Composites 2013, 45, 165–171. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, H.T.; Hsien, K.C.; Lu, C.-Y.; Chena, P.Y. New copper complexes incorporated with the one-step preparation of ionic liquid carbon paste electrode for highly selectively reducing hydrogen peroxide. Electrochem. Commun. 2014, 40, 38–41. [Google Scholar] [CrossRef]
- Tzeng, Y.-W.; Lin, C.J.; Nakano, M.; Yang, C.I.; Wan, W.L.; Lai, L.L. A semi-flexible aminotriazine-based bis-methylpyridine ligand for the design of nickel(II) spin clusters. Dalton Trans. 2014, 43, 3044–3047. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the prepared compounds 4, 6, and 8 are available from the authors.











| Inhibitor | Polarization Method | EIS Method | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Conc. (ppm) | Ba (mV) | Bc (mV) | Ecorr (V) | icorr μA/cm2 | IE% | Rp Ohm | Cdl (μF/cm2) | IE% | |
| Blank | 69 | 120 | −0.3955 | 839 | ____ | 1.80 | 334 | ____ | |
| T3Q (4) | 50 | 55 | 131 | −0.3337 | 55 | 93.4 | 28.5 | 109 | 93.6 |
| 150 | 50 | 187 | −0.3271 | 53 | 93.6 | 30 | 108 | 94.0 | |
| 250 | 77 | 103 | −0.3463 | 14 | 98.8 | 100 | 90 | 98.2 | |
| T3EA (6) | 50 | 98 | 631 | −0.3315 | 557 | 33.6 | 2.8 | 198 | 35.6 |
| 150 | 79 | 413 | −0.3357 | 299 | 64.3 | 5.2 | 153 | 65.3 | |
| 250 | 63 | 174 | −0.3352 | 125 | 85.1 | 13 | 125 | 86.1 | |
| T3AMPy (8) | 50 | 56 | 170 | −0.3324 | 63 | 92.4 | 24 | 112 | 92.5 |
| 150 | 53 | 105 | −0.3382 | 36 | 95.7 | 46 | 105 | 96.0 | |
| 250 | 50 | 112 | −0.3348 | 34 | 96.9 | 47.5 | 104 | 96.2 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Faham, A.; Dahlous, K.A.; AL Othman, Z.A.; Al-Lohedan, H.A.; El-Mahdy, G.A. sym-Trisubstituted 1,3,5-Triazine Derivatives as Promising Organic Corrosion Inhibitors for Steel in Acidic Solution. Molecules 2016, 21, 436. https://doi.org/10.3390/molecules21040436
El-Faham A, Dahlous KA, AL Othman ZA, Al-Lohedan HA, El-Mahdy GA. sym-Trisubstituted 1,3,5-Triazine Derivatives as Promising Organic Corrosion Inhibitors for Steel in Acidic Solution. Molecules. 2016; 21(4):436. https://doi.org/10.3390/molecules21040436
Chicago/Turabian StyleEl-Faham, Ayman, Kholood A. Dahlous, Zeid A. AL Othman, Hamad A. Al-Lohedan, and Gamal A. El-Mahdy. 2016. "sym-Trisubstituted 1,3,5-Triazine Derivatives as Promising Organic Corrosion Inhibitors for Steel in Acidic Solution" Molecules 21, no. 4: 436. https://doi.org/10.3390/molecules21040436
APA StyleEl-Faham, A., Dahlous, K. A., AL Othman, Z. A., Al-Lohedan, H. A., & El-Mahdy, G. A. (2016). sym-Trisubstituted 1,3,5-Triazine Derivatives as Promising Organic Corrosion Inhibitors for Steel in Acidic Solution. Molecules, 21(4), 436. https://doi.org/10.3390/molecules21040436