Abstract
The species Deguelia utilis and Deguelia rufescens var. urucu, popularly known as “timbó,” have been used for many years as rotenone sources in insecticide formulations. In this work, a method was developed and validated using a high-performance liquid chromatography-photodiode array (HPLC-PDA) system, and results were analyzed using hierarchical cluster analysis (HCA). By quantifying the major rotenoids of these species, it was possible to establish a linear relation between them. The ratio between the concentrations of rotenone and deguelin for D. utilis is approximately 1:0.8, respectively, while for D. rufescens var. urucu it is 2:1. These results may help to distinguish these species contributing to their taxonomic identification.
1. Introduction
The roots of some leguminous species from the Derris, Lonchocarpus, Tephrosia, and Deguelia genera have been extensively used as extracts or even in situ in formulations for insecticide, pesticide, and ichthyotoxic purposes [1]. Two Amazonian species have played an important role in the northern region of Brazil: Deguelia utilis (A.C.Sm.) A.M.G.Azevedo, whose basionym is Lonchocarpus utilis A.C.Sm. and synonym is Derris nicou Macbr. (“white timbó”), and Deguelia rufescens var. urucu (Ducke) A.M.G.Azevedo, whose basionym is Derris urucu (Killip & Sm.) J.F. Macbr. and synonym is Lonchocarpus urucu Killip & A.C.Sm. (“red timbó”) [2]. Both species have been useful for local indigenous and traditional peoples due to their ichthyotoxic effects [3].
Recently, several flavonoids and stilbenes have been isolated from the leaves of D. utilis and D. rufescens var. urucu [4,5]. Although some of these substances have shown antioxidant, neuroprotective, and hypoglycemic activities [4,5,6], it is the roots of these species that are the most used part of these plants in many countries, as pesticides and insecticides, and in applications in the organic agriculture sector [7]. Their biosynthetic markers and active principles are rotenoids, especially rotenone and deguelin, at different levels depending on the species [7,8].
Independent studies developed by Cabras et al. [9] and Sherer et al. [10] have called into question the safety of rotenone. The first study reported the persistence of rotenone in olive crops, with half-lives of four days; in the second, the authors administered this substance to rats, with results suggesting that it can cause the same symptoms as Parkinson’s disease (PD), resulting in considerable discussion that culminated in the abandonment of rotenone-based pesticides [9,10]. Hancock et al. [11] examined the relationship between pesticide exposure and the risk of acquiring PD, comparing insecticide and herbicide classes under different conditions of pesticide application. According to these authors, two specific insecticide classes, organochlorines and organophosphorus compounds, can significantly increase PD risk [11]. It seems like the problem is not exactly the rotenone-based pesticide itself, since synthetic ones showed superior risk for acquiring PD, but exposure to any pesticides during their application, because of the failure to use protective equipment [12]. Isman defends the development and use of known botanical pesticides such as rotenone instead of screening for more plants and bioactives or use of synthetic ones [12], given the numerous advantages in reuptake of botanical pesticides such as the low cost and availability of these plants in tropical countries. The use of rotenone-based plants (D. rufescens var. urucu and D. utilis) as pesticides appears to continue being a viable option especially in developing countries such as Brazil [13,14], if protective equipment is used during application.
Although D. rufescens var. urucu and D. utilis have been widely utilized as inputs for preparing pesticide extracts, there has always been considerable divergence in taxonomic classification of these species as well as difficulty in correctly identifying specimens in the field [2,15]. This difficulty may be related to the similarities between them and to morphological changes brought about by factors such as soil, ultraviolet radiation (UV), humidity, or age of the plant [16]. In the specific case of “white timbó” (D. utilis), this difficulty is even greater due to the almost complete lack of flowers as a result of its propagation, having been done using stakes over a long period [17].
Knowledge regarding the chemical composition of botanical species has been widely used for quality control of the botanical material and the products obtained from it and for botanical classification, and may contribute in some cases to the relocation of some species to other genera or even different families of plants [18].
The aim of this work is to quantify rotenone and deguelin using a high-performance liquid chromatographic system with a diode array detector (HPLC-PDA) on the roots of D. rufescens var. urucu and D. utilis and, from these data, provide the basis for better differentiation between them using hierarchical cluster analysis (HCA). The results were compared using regression analysis in order to verify the existence of a relation between concentrations of rotenone and deguelin and confirm the identification of these species.
The method used for this purpose, when compared with those described in the literature, can be considered as rapid, with an analysis time of less than ten minutes [19], as simple, because it uses an isocratic mode of elution and does not use acid or buffer solutions [7,19] and cheaper, since it uses a UV detector instead of a mass detector [20]. Although a mass detector is more sensitive than a UV detector, the latter may well be usefully employed in this case, since the rotenoids have excellent chromophoric groups that allow them to be highly detectable. Furthermore, the method developed here takes into account two markers, rotenone and deguelin, instead of one [21], making it possible to establish a relationship between the concentrations of the two rotenoids in question.
2. Results
2.1. Validation of the HPLC Methodology
2.1.1. Selectivity
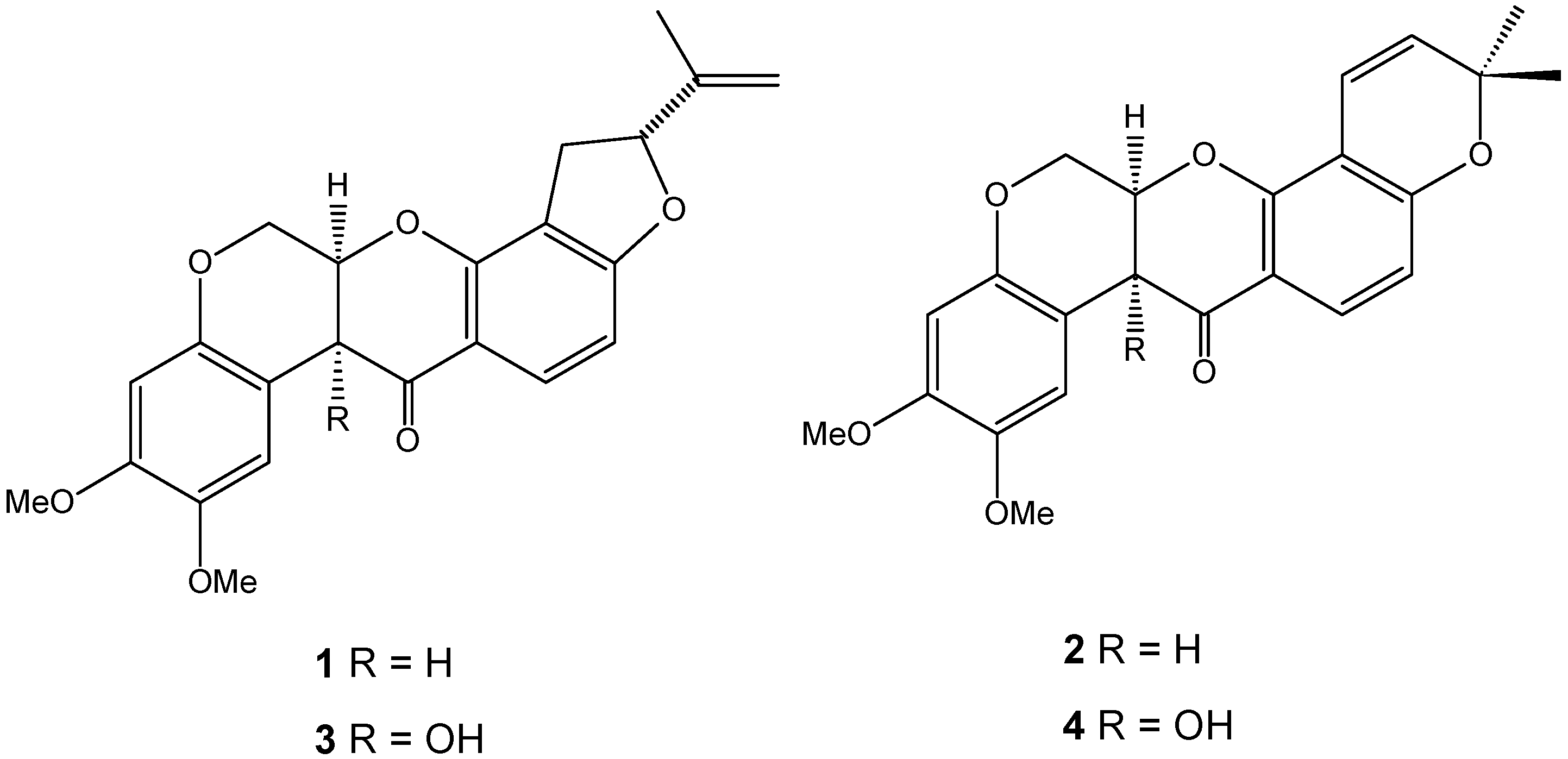
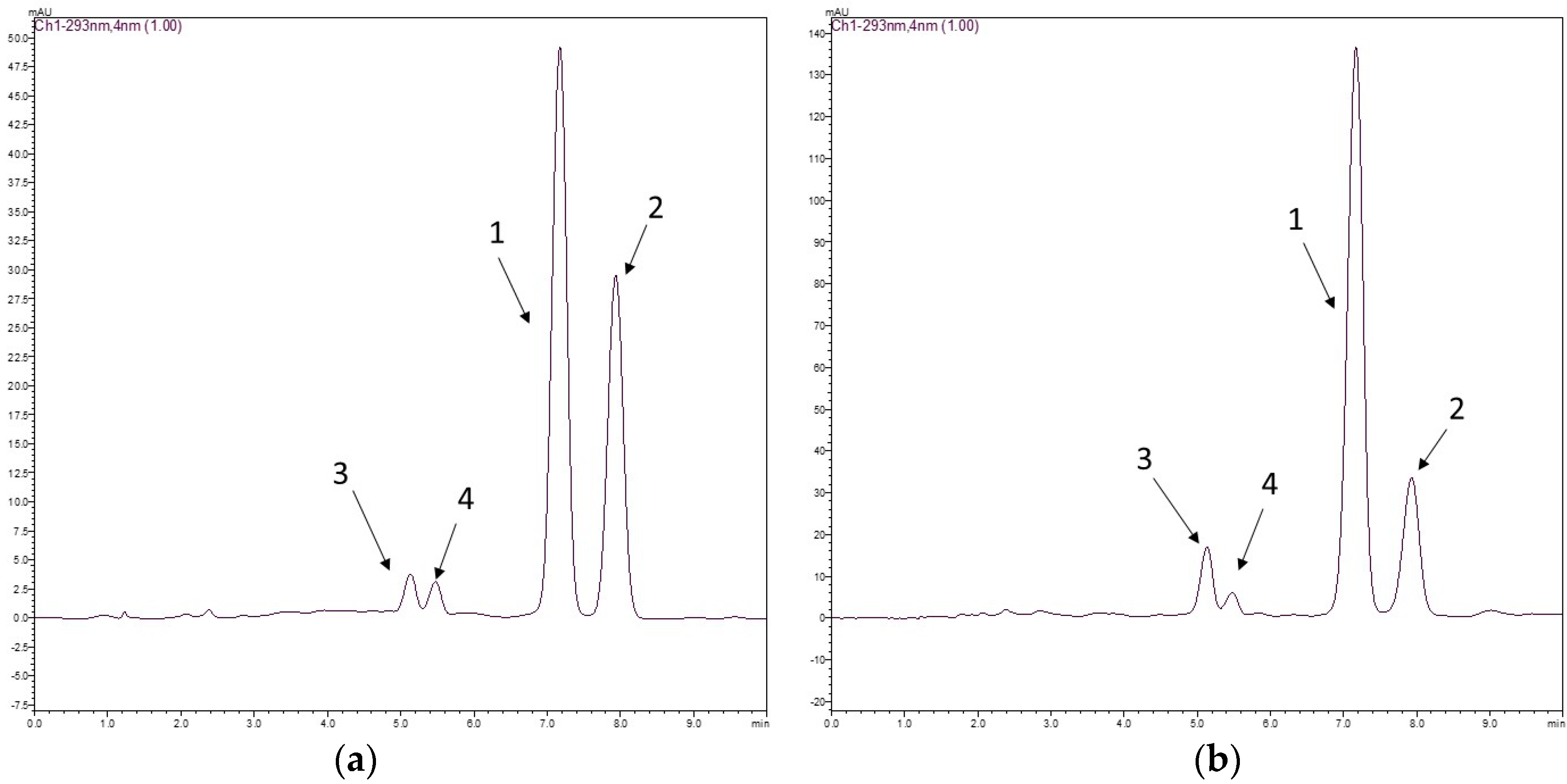
The chromatographic method had good selectivity, and the chromatograms of the two species displayed two clear peaks without any interference. The average elution times of rotenone (1) and deguelin (2) were 7.2 and 8.2 min, respectively. Rotenolone (3) and tephrosin (4) were also observed at 5.2 and 5.6 min, respectively. Figure 1 shows the structures of rotenoids, while Figure 2 shows representative HPLC-PDA chromatograms of the extracts of (a) D. utilis roots and (b) D. rufescens var. urucu roots.
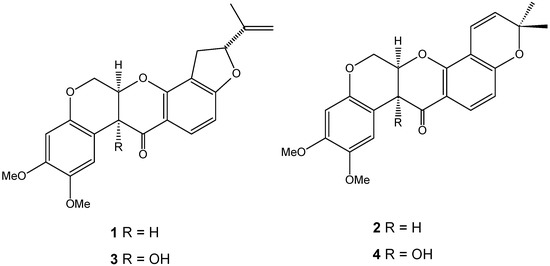
Figure 1.
Structures of rotenoids: 1 rotenone, 2 deguelin, 3 rotenolone and 4 tephrosin.
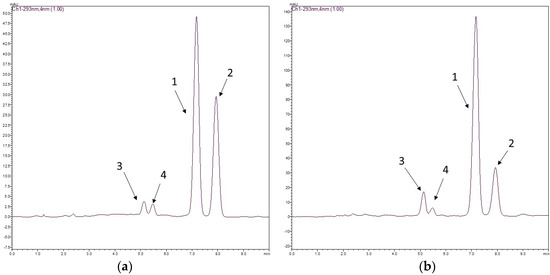
Figure 2.
Typical chromatograms of D. utilis roots extract (a) and D. rufescens var. urucu roots extract (b) for determination of rotenone 1 and deguelin 2. Chromatographic conditions used: a mixture of acetonitrile/water (60:40; v/v) as a mobile phase and as stationary phase a Gemini® C18 column (150 × 4.6 mm, 5.0 μm). The injection volume was 20 μL, and the flow rate 1.0 mL min−1 at 293 nm.
2.1.2. Linearity
The calibration curves were linear within the concentration range assayed. The linearity (r2), regression equation, the limit of detection (LOD), and the limit of quantitation (LOQ) values of each analyte are shown in Table 1.

Table 1.
Linearity (r2), regression equation, limit of detection (LOD), and limit of quantitation (LOQ) values of rotenone and deguelin.
2.1.3. Precision and Accuracy
Precision and accuracy were tested using the quality control (QC) solutions and are presented in Table 2.

Table 2.
Intraday and interday precisions and accuracies data.
2.2. Determination of Concentrations of Rotenone and Deguelin
After development and validation, the method was applied. All roots samples were submitted to ultrasonic extraction and were injected after filtration (20 μL). Rotenone and deguelin concentrations in ten samples of D. utilis (DU-1 to DU-10) and twelve samples of D. rufescens var. urucu are summarized in Table 3.

Table 3.
Concentrations of rotenone and deguelin in the species Deguelia utilis and D. rufescens var. urucu. Values presented in μg of rotenoids per gram of DGR ± CV a.
2.3. Hierarchical Cluster Analysis
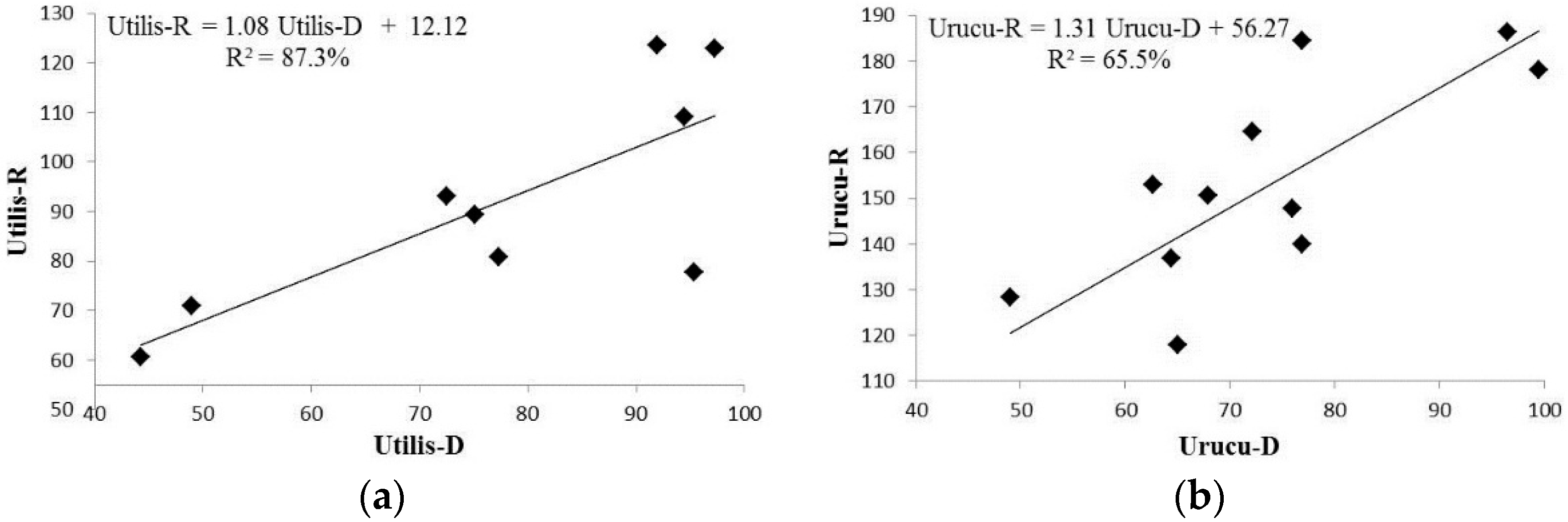
The relationship between the concentration of rotenone and deguelin in the species studied can be accessed through the correlation coefficient, and the regression model established for each species is as shown in Figure 3, where we can observe a strong correlation between the concentrations of rotenone and deguelin in D. rufescens var. urucu (Urucu-R and Urucu-D, respectively), as well as for the same rotenoids in D. utilis (Utilis-R and Utilis-D, respectively).
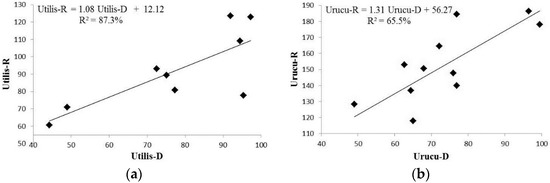
Figure 3.
Regression model for both species with respective equation, wherein (a) Utilis-R and Utilis-D correspond to rotenone and deguelin concentrations on D. utilis roots samples, respectively; and (b) Urucu-R refers to rotenone, and Urucu-D to deguelin concentrations on D. rufescens var. urucu root samples.
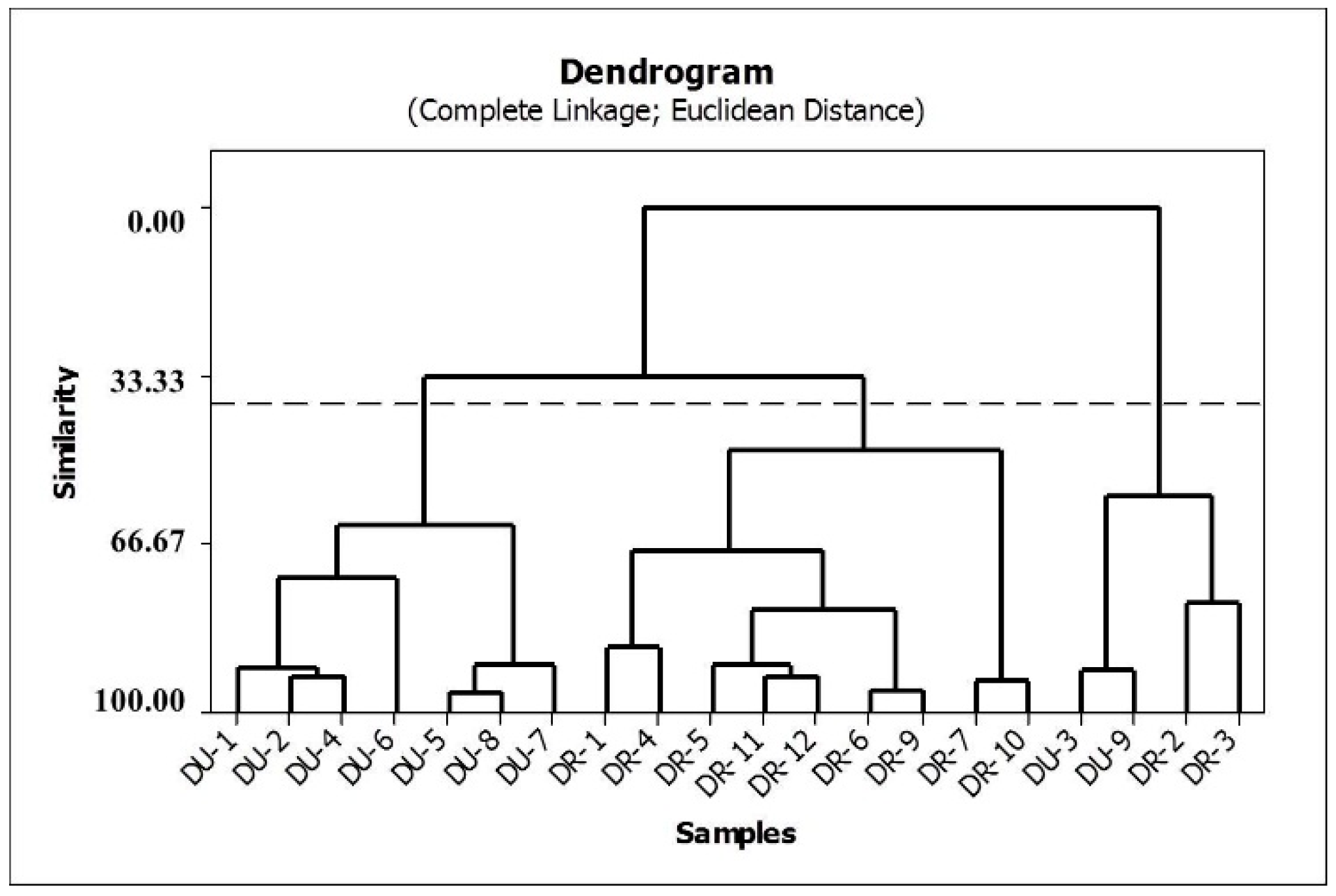
HCA was applied to the auto-scaled data, and the Euclidean distance with the complete linkage method was used to calculate the sample similarities. A hierarchical agglomerative procedure was employed to establish cluster. The results are shown in the dendrogram in Figure 4. In this graph, vertical lines represent roots samples, and horizontal lines represent similarities between samples in terms of the Euclidean distances, which originate from the cluster analysis between pairs of samples, between a sample and a group of samples, and between groups of samples.
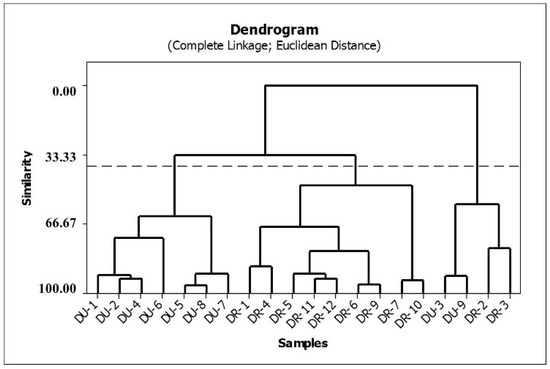
Figure 4.
Dendrogram of similarities between production of rotenone and deguelin in the species.
3. Discussion
These results demonstrate that the production of deguelin increases linearly with rotenone production in accordance with the equation (Utilis-R = 1.08 Utilis-D + 12.12) for D. utilis (Figure 3a) and (Urucu-R = 1.31 Urucu-D + 56.27) for D. rufescens var. urucu (Figure 3b). Such equations make it possible to estimate the concentration of one of the rotenoids (rotenone or deguelin) by quantifying the other one.
According to Semmar et al. [22], similarities or positive correlations can be interpreted in terms of analogies between chemical structures, synchronic metabolisms, or the co-evolution of two compounds under certain environment conditions. Thus, these positive correlations can translate in terms of metabolism and precursor-product relationships between compounds, while negative correlations can be indicative of competitive processes between two compounds for a common precursor(s), enzyme(s), or substrate(s) [23].
The HCA method classified the 21 valid samples studied into three principal groups. The first group comprises the samples of D. utilis, and the second group is composed of the root samples of D. rufescens var. urucu. The third group is a mix of D. rufescens var. urucu and D. utilis species. Based on this classification, it can be observed that the concentrations of rotenone and deguelin are responsible for the chemical separation between D. rufescens var. urucu and D. utilis species.
The observed linear proportion observed between the concentrations of rotenone and deguelin in the Deguelia species studied may be associated with this close biosynthetic relationship between these substances [24].
4. Materials and Methods
4.1. General
Compound analytical standards: Rotenone and deguelin were purchased from Sigma-Aldrich, under codes R8875-1G and D0817-5mg, respectively, with >99% and >98% purity, respectively. Acetonitrile (ACN) (HPLC-grade) and ethyl acetate (analytical grade) were purchased from TEDIA (Fairfield, OH, USA). Ultrapure water was obtained from a Millipore (Molsheim, France) Direct-Q3® system and was used for all experiments. The ultrasonic bath model was Branson 2510 (Danbury, CO, USA).
4.2. Plant Material
The roots of specimens of D. rufescens var. urucu and D. utilis were collected in October 2009 in the EMBRAPA—Amazônia Oriental experimental area located at 01° S 48° W, 10 m, situated in the city of Belém, State of Pará, Brazil. The plants were identified and a voucher of each species (exsiccate IAN 181062 and IAN 181063, respectively) was deposited in the IAN Herbarium from Embrapa—Amazônia Oriental (Belém, Pará, Brazil).
4.3. Calibration Standards and Quality Control Samples
A stock solution of 1000 μg·mL−1 containing the two reference compounds (rotenone and deguelin) was prepared and diluted in acetonitrile to seven appropriate concentrations in order to obtain the working solutions (25; 50; 100; 150; 200; 250; and 300 μg·mL−1). The seven calibration standards were prepared by diluting an appropriate aliquot of the working solutions in acetonitrile (2.5; 5.0; 10.0; 15.0; 20.0; 25.0; and 30.0 μg·mL−1). Three levels of rotenone and deguelin quality control samples were prepared at the concentrations of 3.0 (low), 12.0 (medium), and 24.0 (high) μg·mL−1.
4.4. HPLC Determinations
Analyses were performed using Shimadzu technology (Kyoto, Japan) model LC-20A liquid chromatography fitted with a PDA, SPD-M20A (Shimadzu, Kyoto, Japan). A Phenomenex Gemini® C18 reversed phase analytical column (150 × 4.6 mm I.D., particle size 5.0 μm) was used. The isocratic method was performed for separating rotenone and deguelin using a mobile phase of acetonitrile/water (60:40; v/v) in 8.5 min. The injection volume was 20 μL, and the flow rate was 1.0 mL·min−1. Detection was performed at 293 nm according to satisfactory absorption for both analytes reported in the UV spectrum for rotenone and deguelin. HPLC was controlled and data were elaborated using LC-Solution software (Shimadzu Corporation, Kyoto, Japan, Version 1.25 SP2).
4.5. Validation of Analytical Method
The chromatographic methods were validated to determine the linearity, limit of detection (LOD), limit of quantitation (LOQ), intraday and interday precisions, and accuracies. The precision and accuracy of the analytical method were estimated by intraday and interday analysis of quality control (QC) samples of rotenone and deguelin over a period of three days. QC samples were analyzed in five replicates severally per day to evaluate intraday precision and analyzed over a period of three days for interday precision. During the three days, samples were prepared at high, medium, and low concentrations of five samples each to ensure the analysis was up to standard. The samples were made every day to ensure each batch was new. The precision was calculated by using relative standard deviation (RSD). Accuracy was determined by comparing the concentrations, calculated using calibration curves with known concentrations. The accuracy was expressed as the percentage value of observed concentration to nominal concentration, and the accuracy was expected to be in the range of 85%–115% for each concentration. The RSD should not exceed 15%. The LOD was determined at a signal-to-noise (S/N) ratio of 3, and LOQ was determined at a S/N ratio of 10.
4.6. Preparation of Samples
Samples of 30 cm of roots from 10 specimens of D. utilis (DU-1 to DU-10) and 12 of D. rufescens (DR-1 to DR-12) were accurately collected and cleaned and, after drying, were pulverized and homogenized using a mill (Arno, São Paulo, Brazil). The resulting material was stored in glass vials protected from light and humidity at room temperature until extraction. After exhaustive extraction tests, varying solvents (ethanol, ethyl acetate, acetonitrile, and acetone), the volume of solvent, the amount of roots, the time and the repetition of extraction in ultrasonic bath, and the method for extraction of rotenone and deguelin was optimized (with recovery varying between 101.1% to 104.7% for rotenone and from 103.7% to 106.3% for deguelin in the method chosen). OuvirLer foneticamente Dicionário—Ver dicionário detalhado. Extraction of rotenone and deguelin from the material (5 mg) was carried out four times with 3 mL of ethyl acetate with sonication in an ultrasonic bath for 5 min. Samples were evaporated under vacuum, and the dried extract was accurately dissolved in acetonitrile and filtrated on a 0.45-μm nylon membrane. The solutions were placed in an autosampler vial, and the aliquots (20 μL) were injected into the chromatographic system. Three replicates of each sample were analyzed.
4.7. Hierarchical Cluster Analysis (HCA)
The databases were submitted to the HCA technique, taking into account the concentration of each rotenoid. The HCA examined the distances between the samples in a data set, and the information was then represented in a two-dimensional plot (dendrogram). The most similar points were grouped forming the clusters, and the process was repeated until all the points were inserted into a unique group [25]. All data were statistically analyzed using MINITAB 14.0 software (Minitab Inc., State College, PA, USA).
4.8. Statistical Analysis
Analysis of variance (ANOVA) and regression analysis were performed by MINITAB 14 software (2003). Mean comparisons were performed with Tukey’s test at p ≤ 0.05, when appropriate.
5. Conclusions
All extracts of D. rufescens var. urucu presented greater quantities (approximately double) of rotenone when compared to D. utilis extracts, making the former species a better source of rotenone for pesticide purposes, since this rotenoid is more active than deguelin.
Quantification results show a linear relation between the ratio of rotenone and deguelin production in both species. The concentration of these rotenoids in D. utilis was approximately the same, with deguelin presenting a lower concentration on all samples when compared with rotenone, except for DU-6. This ratio for D. rufescens var. urucu specimens is about twice more rotenone than deguelin.
The analytical method developed by HPLC-PDA together with hierarchical cluster analysis can be used as additional tools for differentiating species, making this study a pathway to distinguishing the studied species.
Acknowledgments
The authors are grateful to Antônio Pedro de S. Filho from EMBRAPA Amazônia Oriental for supplying the plants and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Pará (FAPESPA), and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) for financial support.
Author Contributions
D.F. and A.P. collected plant materials and prepared the samples; M.A. and A.A. contributed to acquisition of equipment and reagents; G.G. provided standards and reviewed the paper; C.A. performed HCA analysis; D.C., C.S., and M.S. conceived, designed the analytical experiments, validated the method, and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HPLC | high-performance liquid chromatography |
| PDA | photodiode array |
| HCA | hierarchical cluster analysis |
| PD | Parkinson’s disease |
| UV | Ultraviolet |
| LOD | limit of detection |
| LOQ | limit of quantitation |
| QC | quality control |
| DR | Deguelia rufescens var. urucu |
| DU | Deguelia utilis |
| ND | not detected |
| RSD | relative standard deviation |
References
- Fukami, H.; Nakajima, M. Naturally Occurring Insecticides; Dekker: New York, NY, USA, 1971. [Google Scholar]
- Tozzi, A.M.G.A. The identity of timbó-verdadeiro: Deguelia utilis (A.C.Sm.) A.M.G. Azevedo (Leguminosae-Papilionoideae). Rev. Bras. Biol. 1998, 58, 511–516. [Google Scholar] [CrossRef]
- Ducke, A. Notas Sobre a Flora Neotrópica—II as leguminosas da Amazônia brasileira. Bolm. Téc. Inst. Agron. N. 1949, 18, 171–200. [Google Scholar]
- Lôbo, L.T.; da Silva, G.A.; Ferreira, M.; da Silva, M.N.; Santos, A.S.; Arruda, A.C.; Guilhon, G.M.S.P.; Santos, L.S.; Borges, R.S.; Arruda, M.S.P. Dihidroflavonols from the leaves of Derris urucu (Leguminosae): Structural elucidation and DPPH radical-scaveging activity. J. Braz. Chem. Soc. 2009, 20, 1082–1088. [Google Scholar] [CrossRef]
- De Oliveira, D.G.; de Almeida, C.M.C.; e Silva, C.Y.Y.; Arruda, M.S.P.; Lopes, D.C.F.; Yamada, E.S.; da Costa, E.T.; da Silva, M.N. Flavonoids from the leaves of Deguelia utilis (Leguminosae): Structural elucidation and neuroprotective properties. J. Braz. Chem. Soc. 2012, 23, 1933–1939. [Google Scholar] [CrossRef]
- Pereira, A.C.; Arruda, M.S.; da Silva, E.A.; da Silva, M.N.; Lemos, V.S.; Cortes, S.F. Inhibition of α-glucosidase and hypoglycemic effect of stilbenes from the Amazonian plant Deguelia rufescens var. urucu (Ducke) A.M.G. Azevedo (Leguminosae). Planta Med. 2012, 78, 36–38. [Google Scholar] [PubMed]
- Cabizza, M.; Angioni, A.; Melis, M.; Cabras, M.; Tuberoso, C.V.; Cabras, P. Rotenone and rotenoids in cube resins, formulations, and residues on olives. J. Agric. Food Chem. 2004, 52, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Cabras, P.; Cabone, P.; Cabras, M.; Angioni, A.; Russo, M. Rotenone residues on olives and in olive oil. J. Agric. Food Chem. 2002, 50, 2576–2580. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Isoflavonoids of the Leguminosae. Nat. Prod. Rep. 2009, 26, 776–802. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [PubMed]
- Hancock, D.B.; Martin, E.R.; Mayhew, G.M.; Stajich, J.M.; Jewett, R.; Stacy, M.A.; Scott, B.L.; Vance, J.M.; Scott, W.K. Pesticide exposure and risk of Parkinson’s disease: A family-based case-control study. BMC Neurol. 2008, 8, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Ismann, M.B. Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.; Ward, A.; McNamara, N.; Denholm, I. Exploring the factors that influence the uptake of botanical insecticide by farmers: A case study of tobacco-based products in Nigeria. Exp. Agric. 2002, 38, 469–479. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides: For richer, for poorer. Pest Manag. Sci. 2008, 64, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Souza Filho, A.P.D.S.; do Nascimento, J.L.M. Timbó: Aspectos Botânicos e Moléculas Bioativas; Embrapa Amazônia Oriental: Belém, Brazil, 2012. [Google Scholar]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quím. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Magalhães, A.F.; Tozzi, A.M.G.A.; Magalhães, E.G.; Moraes, R.S.M. Prenylated flavonoids from Deguelia hatschbachii and their systematic significance in Deguelia. Phytochemistry 2001, 57, 77–89. [Google Scholar] [CrossRef]
- Sartor, C.I.P.; da Silva, M.F.G.F.; Fernandes, J.B.; Vieira, P.C.; Fo, E.R.; Cortez, D.A.G. Alkaloids from Dictyoloma vandellianum: Their chemosystematic significance. Phytochemistry 2003, 63, 185–192. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Bernal, J.L.; del Nozal, M.J.; Novo, M.; Higes, M.; Llorente, J. Determination of rotenone residues in raw honey by solid-phase extraction and high-performance liquid chromatography. J. Chromatogr. A 2000, 871, 67–73. [Google Scholar] [CrossRef]
- Caboni, P.; Sarais, G.; Angioni, A.; Garau, V.L.; Cabras, P. Fast and Versatile Multiresidue Method for the Analysis of Botanical Insecticides on Fruits and Vegetables by HPLC/DAD/MS. J. Agric. Food Chem. 2005, 53, 8644–8649. [Google Scholar] [CrossRef] [PubMed]
- Lautié, E.; Rozet, E.; Hubert, P.; Queting Leclercq, J. Quantification of rotenone in seeds of different species of yam bean (Pachyrhizus sp.) by a SPE HPLC-UV method. Food Chem. 2012, 131, 1531–1538. [Google Scholar] [CrossRef]
- Semmar, N.; Jay, M.; Nouira, S. A new approach to graphical and numerical analysis of links between plant chemotaxonomy and secondary metabolism from HPLC data smoothed by a simplex mixture design. Chemoecology 2007, 17, 139–156. [Google Scholar] [CrossRef]
- Crombie, L.; Whiting, D.A. Review article 135 biosynthesis in the rotenoid group of natural products: Applications of isotope methodology. Phytochemistry 1998, 49, 1479–1507. [Google Scholar] [CrossRef]
- Da Costa, J.P.C.; Bélo, M. Teores de rotenona em clones de timbó (Derris spp., Fabaceae) de diferentes regiões da Amazônia e os seus efeitos na emergência de imagos em Musca domestica L. Acta Amazon. 1999, 29, 563–573. [Google Scholar] [CrossRef]
- Sharaf, M.A.; Illman, D.L.; Kowalski, B.R.; Kirk-Othmer. Encyclopedia of Chemical Technology Chemometrics; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).