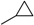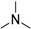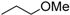Abstract
A library of thirty novel thiazolo[5,4-f]quinazolin-9(8H)-one derivatives belonging to four series designated as 12, 13, 14 and 15 was efficiently prepared, helped by microwave-assisted technology when required. The efficient multistep synthesis of methyl 6-amino-2-cyano- benzo[d]thiazole-7-carboxylate (1) has been reinvestigated and performed on a multigram scale. The inhibitory potency of the final products against five kinases involved in Alzheimer’s disease was evaluated. This study demonstrates that some molecules of the 12 and 13 series described in this paper are particularly promising for the development of new multi-target inhibitors of kinases.
1. Introduction
Major human diseases such as cancer, neurodegenerative disorders and cardiovascular diseases have been closely associated with the deregulation of kinases [1,2,3]. Consequently, protein kinases represent pertinent targets for academic and industrial chemists searching for kinase inhibitors as potential new therapeutic agents [4,5,6]. Most kinases phosphorylate both serine and threonine residues, others phosphorylate tyrosines, and a small number phosphorylate all three amino acids (dual-specificity kinases). Our research groups are mostly invested in the synthesis of sulfur- nitrogen heteroaromatic molecules able to modulate the activity of deregulated kinases thought to be involved in Alzheimer’s disease (AD) [7,8,9,10,11,12,13,14,15]. These five important kinases used in this study are the Ser/Thr kinases (CDK5, GSK-3, CLK1 and CK1) and the dual-specificity kinases (DYRK1A family) [16,17,18,19]. The important impact of these kinases in various key cellular regulatory mechanisms is justifying recent approaches consisting in the design of multi-target-directed ligands (MTDLs) [20,21,22,23] able to target more than one kinase. This highly pertinent therapeutic strategy may allow the development of new tools or therapies to better understand and treat patients suffering of neurodegenerative diseases.
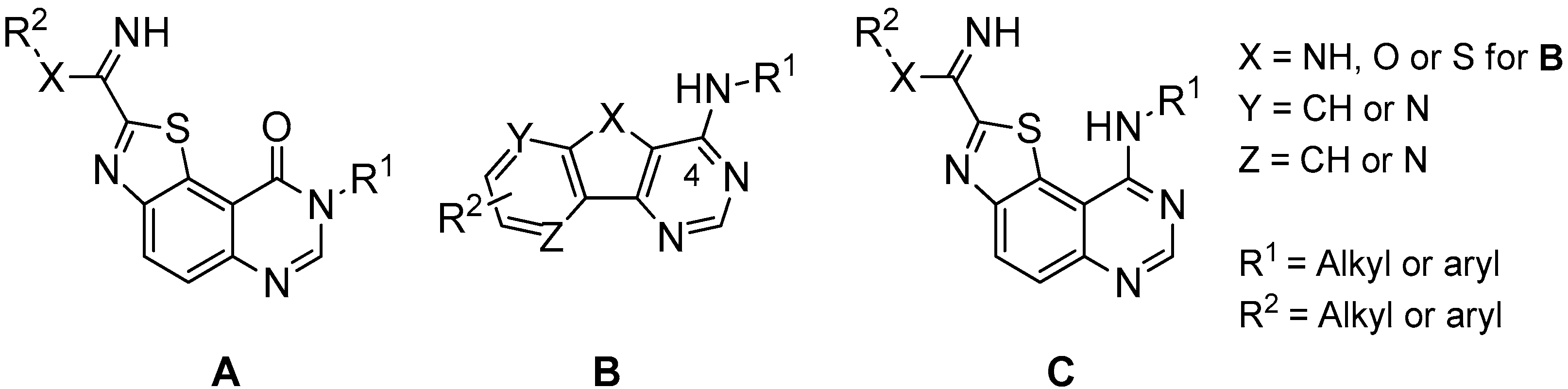
In the course of our work, we previously described the synthesis of a small library of 8H-thiazolo[5,4-f]quinazolin-9(8H)-ones (A in Figure 1) as dual CDK1/GSK-3 kinases inhibitors. Brief studies of their inhibitory potency were realized with a small panel of kinases and showed two compounds (I and II in Figure 2) with a micromolar range inhibitory effect on CDK1 and GSK-3 [7,8]. More recently, the synthesis and the kinase inhibitory potency of various N-arylbenzothieno[3,2-d]pyrimidin-4-amines, and their pyrido and pyrazino analogues (B in Figure 1), have been published. These original heteroaromatics provide new means to target and inhibit some of the abovementioned kinases in the nanomolar range [9,10,11].
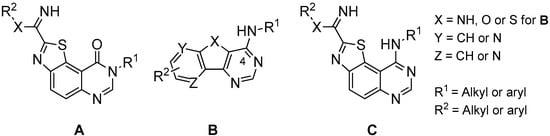
Figure 1.
General formulae A, B and C of previously described kinase inhibitors [6,7,8,9,10,11,12,13,14].
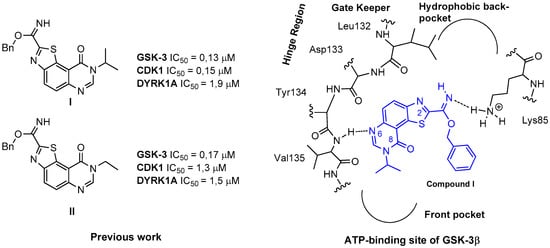
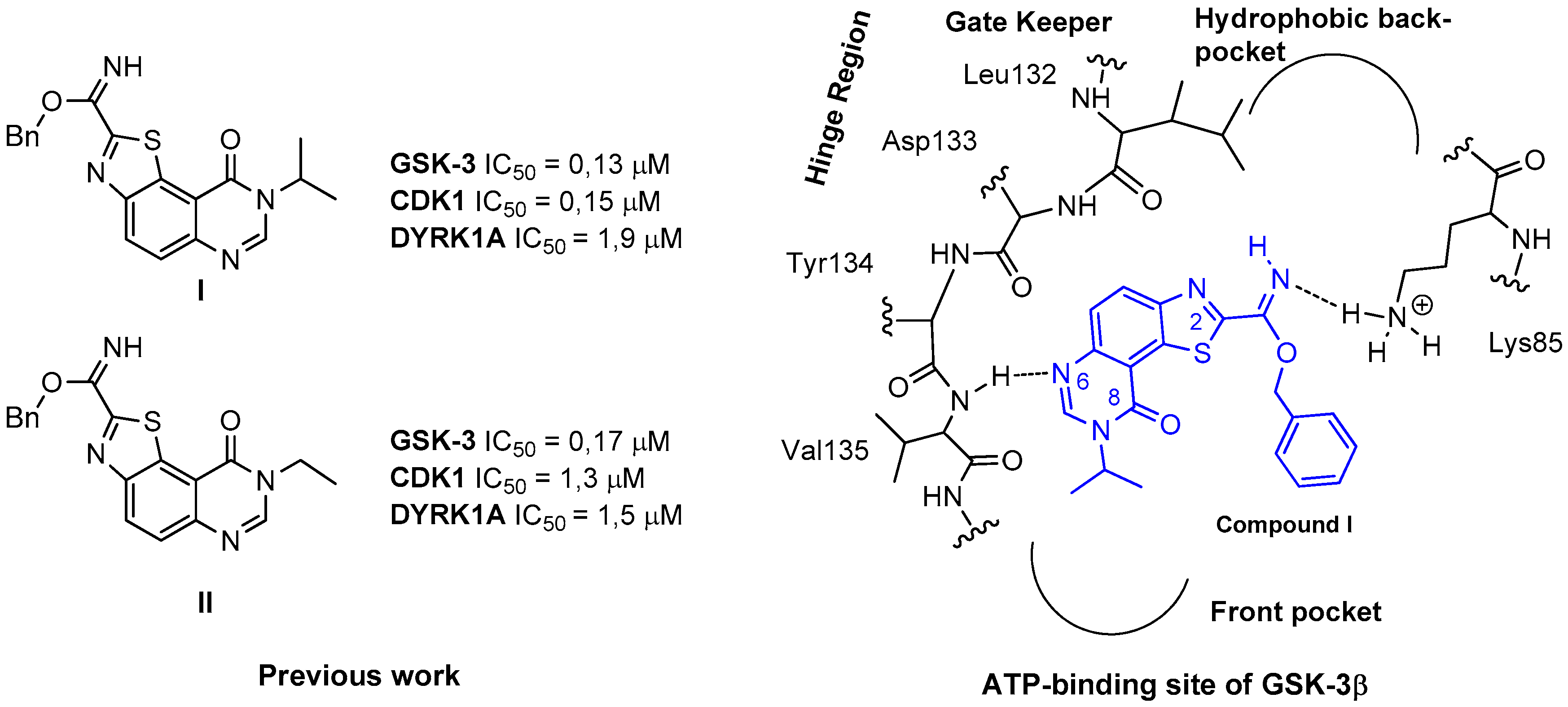
Figure 2.
Schematic representation of the proposed binding mode of compound I in the ATP-binding site of GSK-3β.
The overall pharmaceutical interest of all these compounds encouraged us to conceive new series of thiazolo[5,4-f]quinazolines substituted in position 4 of the pyrimidine ring by an aromatic amine and by carboximidamide or amidine groups in position 2 of the thiazole moiety [6,12,13,14,15] (see general formula C in Figure 1). These compounds were conceived as 6,6,5-tricyclic homologues of the basic 4-aminoquinazoline pharmacophore which is present in approximately 80% of ATP-competitive kinase inhibitors that have received approval for the treatment of cancer [4,5]. Five of the novel thiazolo[5,4-f]quinazoline derivatives prepared displayed single-digit nanomolar or subnanomolar IC50 values and are among the most potent and selective DYRK1A/1B inhibitors disclosed to date [13,14,15].
Returning to our initial work [7,8] and extending the list of targeted kinases, we discovered that compounds I and II exhibit micromolar IC50 values against DYRK1A (Figure 2). This result suggested the possibility to extend the scope of pertinent kinases that these ligands are able to target.
The results of various docking studies realized to understand the structure-activity relationships (SARs) are represented in Figure 2 and concern the ATP-binding site of GSK-3β [7,8,24]. They suggest that the unencumbered nitrogen in position 6 of the tricyclic core can form a hydrogen bond with the backbone NH-residue of Val135 in the hinge segment. This region of kinases ATP-binding site is considered as a critical H-bonded system with the majority of inhibitors that have been published to date. As described in Figure 2, a benzylcarbimidate function may form polar interaction between nitrogen atom of the imidate and the ammonium group of Lys85. In this hypothesis a bulky substituent at the R2 position may not fit into the hydrophobic back-pocket of GSK-3β. This region of kinases ATP-binding site is known to not be conserved among kinases and can thus be used to gain affinity as well as selectivity.
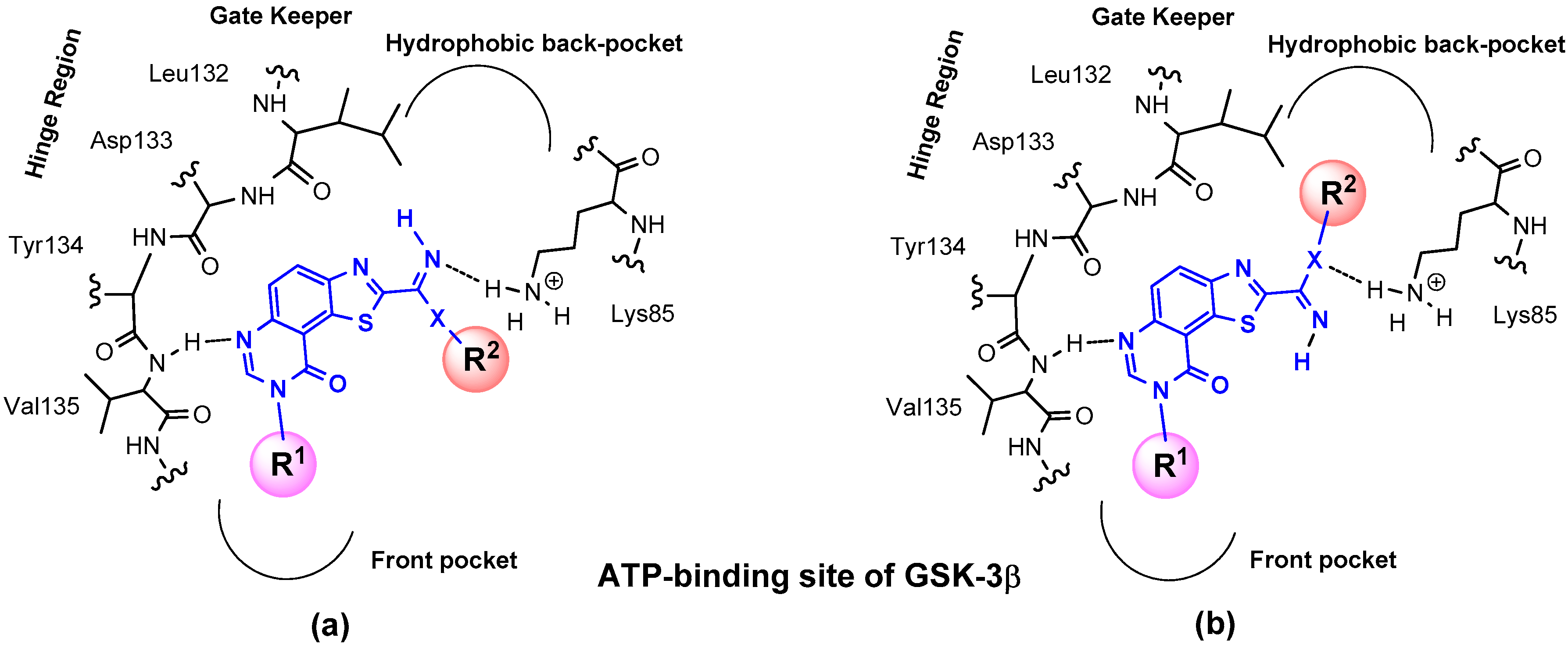
As depicted in Figure 3, modulating the size and/or the nature of R1 and R2 may have an effect on the affinity of the ligands by allowing cis- or trans-spatial positions of these groups in the targeted binding sites. In the latest case, R2 group will be able to fit into the back-pocket of kinases and concomitantly, the R2 group present on N8 may also influence the position of the inhibitor into the enzyme site.
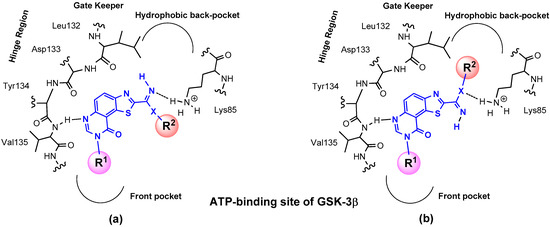
Figure 3.
Suggested position of envisioned molecules in the targeted binding sites (example of the ATP-binding site of GSK-3β: (a) cis- spatial position of R1 and R2 or (b) trans-spatial position of R1 and R2.
Considering all these facts, the synthetic route to the thiazolo[5,4-f]quinazolin-9(8H)-one scaffold was optimized with the aim of modulating the R1 and R2 groups. This was expected by substituting the position 8 of the pyrimidine ring with various alkyl and aryl groups and by introducing various alkyl substituents on the carbimidate groups in position 2 of the thiazole moiety. Concerning this last point, the choice of the aliphatic chains for R1 was inspired by previous results (see compounds C in Figure 1) showing that small size groups can help to enhance the inhibitory activity against kinases [13].
This paper describes the development of a simple and reliable method allowing the preparation of a library of new thiazolo[5,4-f]quinazolin-9(3H)-ones for which interesting multi-target kinase inhibitory activities were observed. The main part of the chemistry described in this paper was achieved under microwave irradiation as a continuation of our global strategy consisting in the design of appropriate reagents and techniques offering operational, economic, and environmental benefits over conventional methods [25,26,27].
2. Results and Discussion
2.1. Chemistry
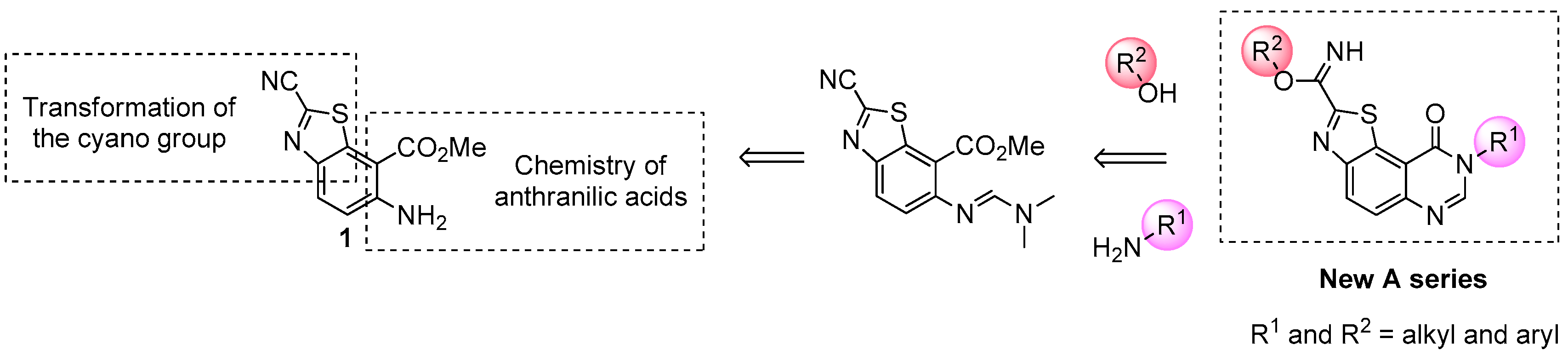
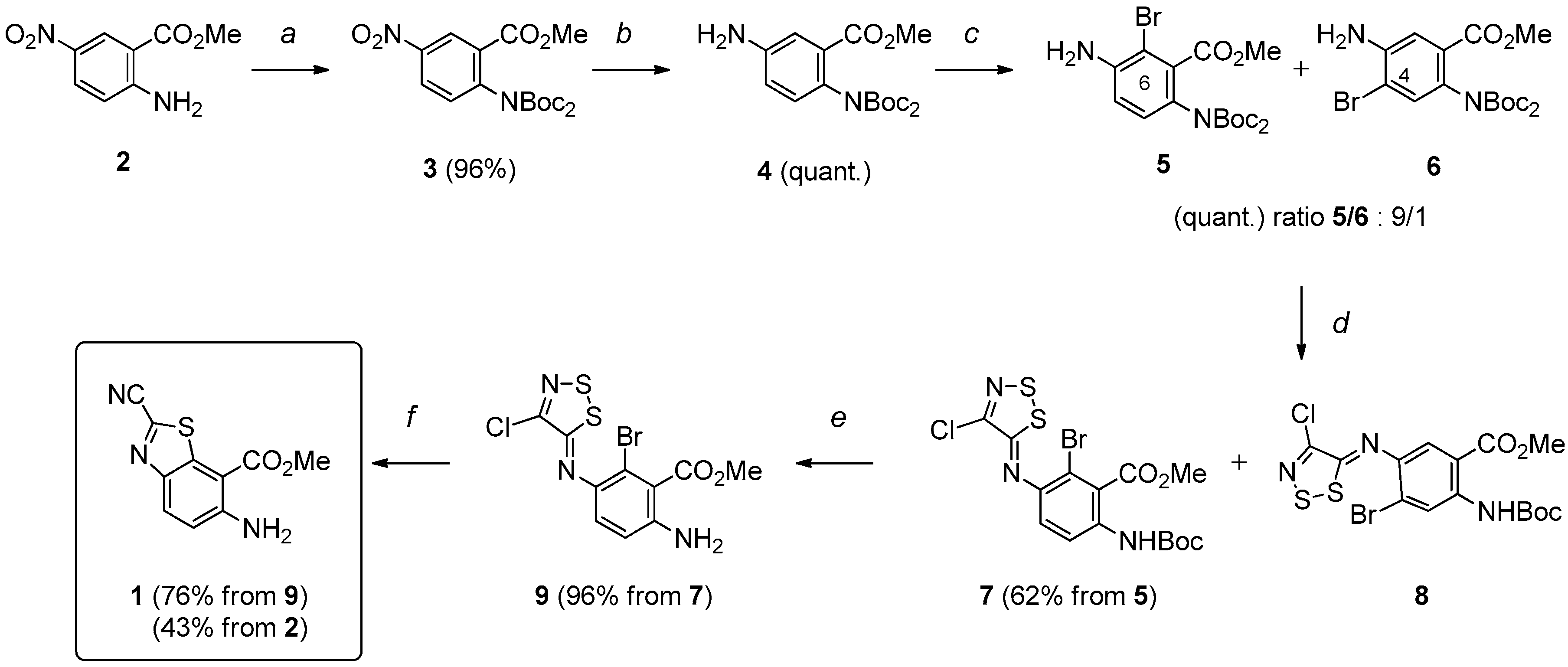
The target molecules were thiazolo[5,4-f]quinazolin-9(8H)-ones substituted in position N8 (which corresponds to position 4 of the pyrimidine ring) by an aliphatic chain or an aromatic substituent (Scheme 1). In order to reach an efficient route to these various 8-substituted thiazolo[5,4-f]quinazolin-9(8H)-ones, a rational multistep synthesis of a novel polyfunctionalized benzothiazole (see 1 in Scheme 1) has been developed [28,29,30]. This molecular system was conceived to be a versatile efficient precursor to various target molecules. The presence of the carbonitrile function in position 2 of the thiazole ring should allow easy access to (alkyl)carbimidate function and the ortho-aminobenzoïc ester part should access to the target N8-substituted pyrimidin-4-one derivatives.
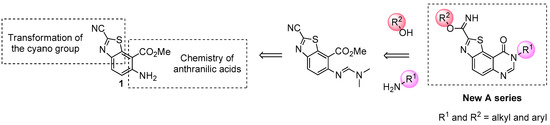
Scheme 1.
Envisioned transformations of 1 for synthesis of novel compounds of series A.
Based on our previous studies [25,26,27], the synthesis of the key intermediate 1 was revised and optimized in six steps according to the procedure depicted in Scheme 2. N2-Protection of methyl 5-nitroanthranilate (2) [31] provided methyl 2-[di(tert-butoxycarbonyl)amino]-5-nitrobenzoate (3), which was reduced by treatment with ammonium formate in the presence of a catalytic amount of 10% palladium charcoal. The resulting aromatic amine (4) was treated with N-bromosuccinimide (NBS) in DMF to give 5 and 6 (ratio 5/6: 9/1) in a quantitative yield. The mixture of ortho-bromo anilines 5 and 6 was reacted with Appel’s salt (4,5-dichloro-1,2,3-dithiazolium chloride) to give intermediate imino-1,2,3-dithiazoles 7 and 8 which were separated and purified by flash-column chromatography on silica gel. The intermediate imine 7 was transformed into the target methyl 6-amino-2-cyanobenzo[d]thiazole-7-carboxylate (1) after N2-deprotection (giving 9, 96%) and microwave-assisted copper-mediated cyclization. This synthetic route allowed an efficient and reproducible preparation of 1, in a good overall yield of 43%, helped in some steps by microwave-assisted heating. In terms of efficiency, 20 g of 2-methyl 5-nitroanthranilate (2) may lead to 6 g of polyfunctionalized benzo[d]thiazole 1.
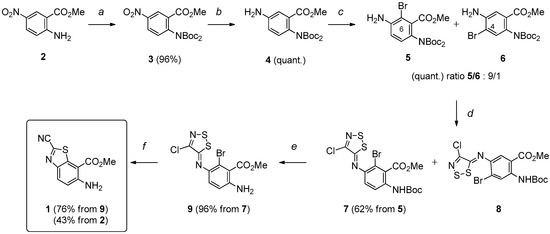
Scheme 2.
Multistep synthesis of the key benzothiazole 1. Reagents and conditions: (a) Boc2O (2.3 equiv), DMAP (1.0 equiv), Et3N (1.0 equiv), THF, r.t., 5 h; (b) HCO2NH4 (5.0 equiv), Pd/C (10%), EtOH, 78 °C (μw), 15 min; (c) NBS (1.0 equiv), DMF, −10 °C, 3 h; (d) Appel salt (1.2 equiv), Py. (2.3 equiv), CH2Cl2, r.t., 3 h; (e) TFA, CH2Cl2, r.t., 2 h; (f) CuI (1.0 equiv), Py., 115 °C (μw), 30 min.
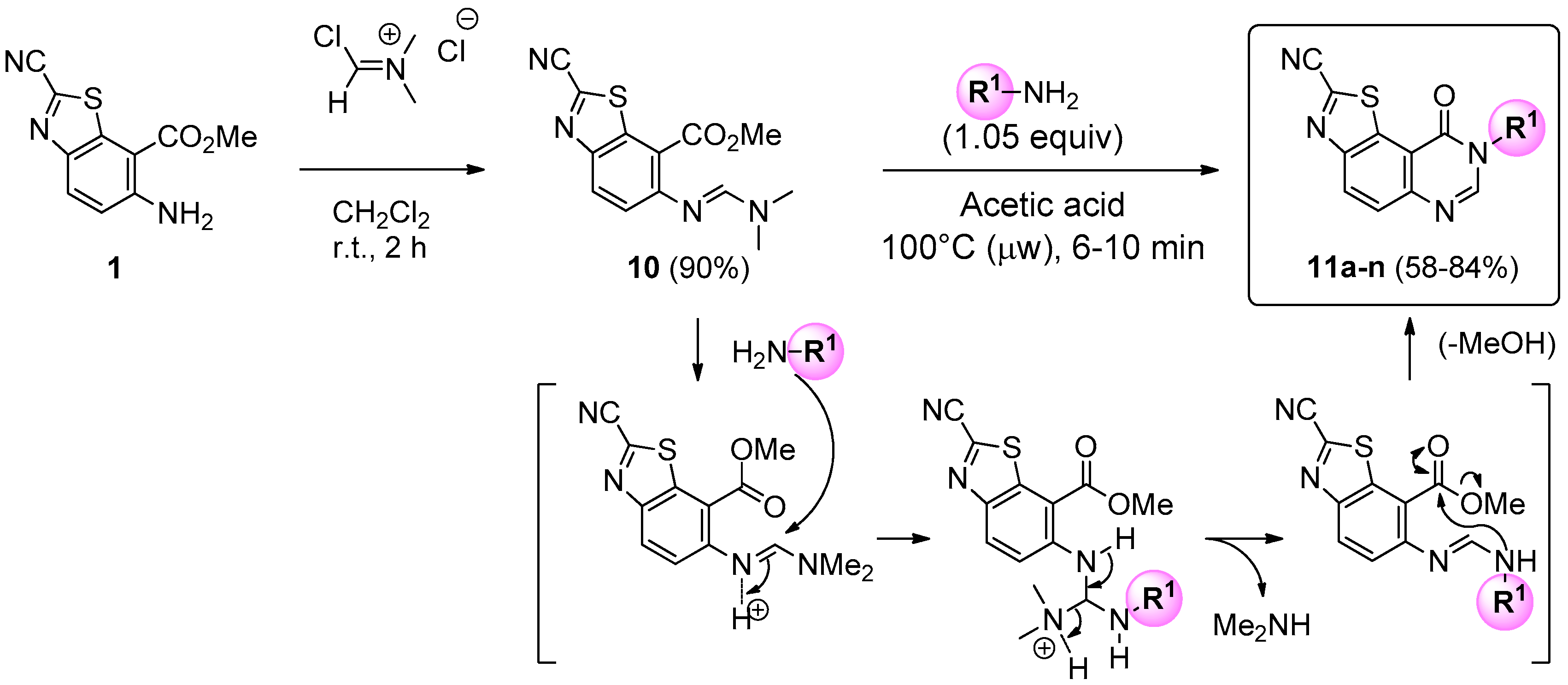
Treatment of 1 with 1.5 equiv of Vilsmeier-Haack reagent in dichloromethane at room temperature gave (E)-methyl 2-cyano-6-([(dimethylamino)methylene]amino)benzo[d]thiazole-7-carboxylate (10) in excellent yield (90%). This efficient synthesis can be performed at the multi-gram scale, enabling preparation of several grams of the key precursor 10. The aforementioned formimidamide was heated at 100 °C under microwave irradiation, in the presence of 1.05 equiv of appropriate amines, in acetic acid. After irradiation times of 6 to 10 min, cyclization of the pyrimidin-4-one part of the tricyclic product allowed access to the expected N8-substituted-9-oxo- 8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitriles (series 11) in moderate to good yields (58%–84%). Considering previous kinetics studies [32], it may be assumed that the mechanism of cyclization occurred via a first attack of the amine on the activated carbon of the amidine. The intermediate triamine species released dimethylamine and cyclized into the expected quinazolin-4-one derivatives (Scheme 3).
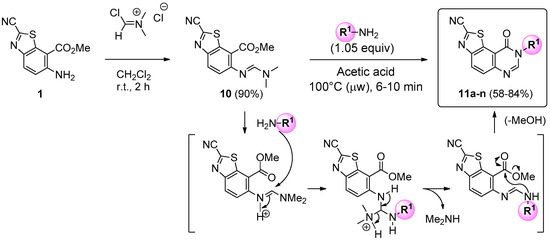
Scheme 3.
Synthesis of series 11 compounds and suggested mechanism of cyclization after attack of the primary amine. For yields see Table 1.
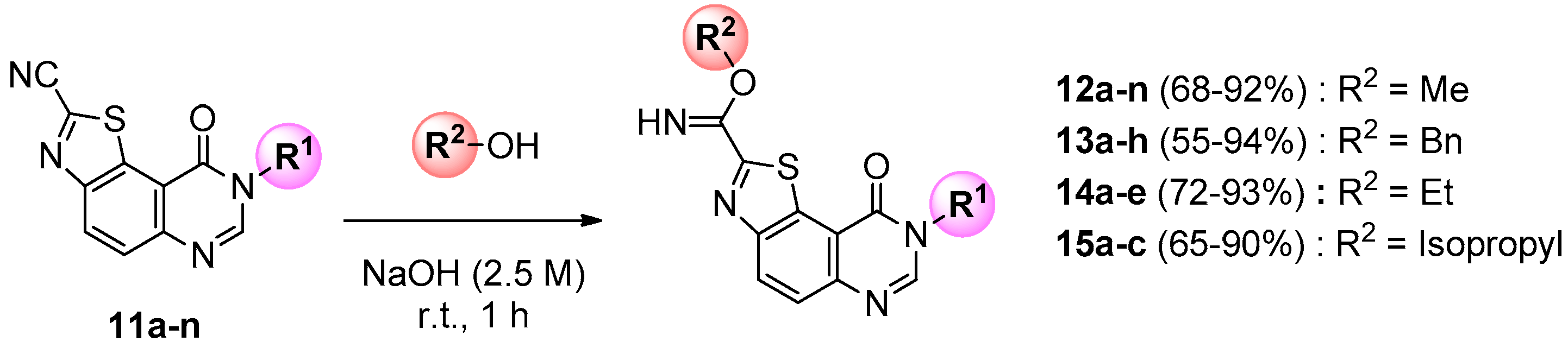
At the last stage of the synthesis, transformation of the carbonitrile group into a methylcarbimidate was realized by stirring compounds 11a–n with sodium hydroxide (2.5 M in water) in methanol at room temperature for 1 h (Scheme 4). Methyl 9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidates (12a–n) were thus obtained in good to excellent yields (Table 2). In the course of SAR studies, libraries of various carbimidates were extended to ethyl, isopropyl, and benzyl derivatives using the same procedure and the appropriate alcohol (Scheme 4).
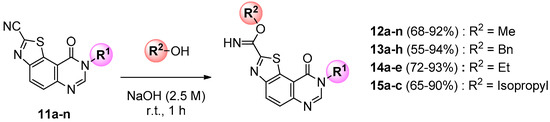
Scheme 4.
General synthesis of carbimidates 12, 13, 14 and 15 from 11 series (for yields see Table 2).

Table 2.
Synthesis of carbimidates 12, 13, 14 and 15 from 11 series.
The synthesis of forty-four 8-susbtituted thiazolo[5,4-f]quinazolin-9(8H)-one derivatives was performed with success. This process was helped by the use of methyl 6-amino-2-cyano- benzo[d]thiazole-7-carboxylate (1) [27], a molecular platform conceived to be a versatile and efficient precursor to various target molecules. Note that microwave heating was mainly performed at atmospheric pressure in a controlled multimode cavity with a microwave power delivery system ranging from 0 to 1200 W. Concerning the technical aspects, open-vessel microwave experiments have some advantages, such as the possibility of easier scale-up and the use of common laboratory glassware.
2.2. Biological Studies
Products of series 11a–c, 12a–n, 13a–h, 14a–e and 15a–c were tested in five different in vitro kinase assays (CDK5/p25 (cyclin-dependent kinase), CK1δ/ε casein kinase 1), GSK-3α/β glycogen synthase kinase 3), DYRK1A (dual-specificity, tyrosine phosphorylation regulated kinase) and CLK1 (cdc2-like kinase 1) to evaluate their inhibition potency [33,34,35,36,37]. All compounds were first tested at a final concentration of 10 μM. Compounds showing less than 50% inhibition were considered as inactive (IC50 >10 μM). Compounds displaying more than 50% inhibition at 10 μM were next tested over a wide range of concentrations (usually 0.01 to 10 μM), and IC50 values were determined from the dose-response curves (Sigma-Plot). Harmine (Table 3) is a β−carboline alkaloid known to be a potent inhibitor of DYRK1A [38]. It was also tested as positive control and its IC50 values were compared to those obtained for the compounds under study.

Table 3.
Kinase inhibitory activity a,b of the thiazolo[5,4-f]quinazoline series 11a–c, 12a–n, 13a–h, 14a–e, 15a–c.
Results given in Table 3 demonstrate that none of the tricyclic derivatives prepared in this work showed significant inhibitory activity against CDK5/p25 and CK1δ/ε. Only three compounds of 11a–c series are described in Table 3 and none of them showed any significant activity against the targeted kinases. These results are consistent with previous studies [7,8].
The results of the kinases inhibition potential obtained with compounds of the 14a–e and 15a–c series were also quite disappointing. Apart from product 14e, which exhibits a good and selective inhibition of GSK-3 (IC50 = 0.029 μM) and product 15a, which revealed a fairly good inhibition of CLK1 (IC50 = 0.043 μM), the IC50 values obtained for the other compounds of these two series (14a–d, 15b and 15c) were not significant.
In general, interesting and significant biological activity of the tested compounds was oriented towards three kinases of the initial panel: CLK1, DYRK1A and GSK-3α/β.
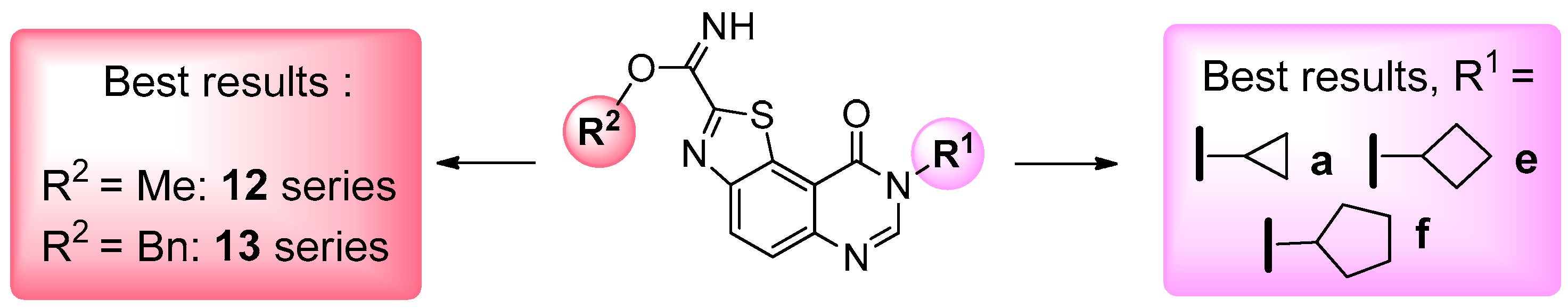
Undoubtedly, the most active molecules prepared in this study were series 12 and 13 in which the final carbimidate function was obtained after attack of methanol or benzyl alcohol. Most of these compounds from these two series showed submicromolar activities against CLK1, DYRK1A and GSK-3α/β, except product 12m which was completely inactive (IC50 > 10 μM for the five kinases tested). Curiously, despite the size modification of the carbimidate substituents, similar activity profiles can be observed for the 12 and 13 series (see Table 3 in which the most significant results are underlined in grey and significant IC50 values in the nanomolar range written in bold). It appears that the most significant results were obtained with compounds of the a and d–g series in the two family products (12 and 13).
Results obtained with compounds 12h–l (CLK1: 0.691 μM < IC50 < 7.9 μM), DYRK1A (0.2 μM < IC50 < 3.0 μM) and GSK-33 α/β (0.3 μM < IC50 < 1.8 μM) demonstrated the necessity to conserve a cyclic alkyl group in position 8 of the tricyclic core (12a and 12d–g), in order to maintain good to very good affinity. The presence of basic groups in N8 (12h–j) may be correlated with an rather important decrease of affinity, in the same way as the presence of aromatic substituents in position 8 of the thiazolo[5,4-f]quinazolin-9(8H)-one core (compounds 12k–m). In this last case, it appeared that the nature of the substituents present in the para-position of the phenyl group in N8 may influence and decrease the IC50 values measured for the various kinases. Compared with data obtained for compounds in the 12d–g series, it seems to be the consequence to steric influence rather than electronic effects. Compounds 12b, 12c and 13b, 13c were also analyzed and although their inhibitory activity towards the three kinases (CLK1, DYRK1A and GSK-3α/β) was characterized by submicromolar values of IC50, their global activity was considered not significant enough to continue further studies.
Comparison of the substituents present on N8 position of a and d–f series shows mainly a small-size ring (e.g., cyclopropyl for 12a and 13a series) or its equivalent such as isopropyl (12d and 13d), cyclobutyl (12f and 13f) and cyclopentyl (12e and 13e). The size of the cycle present in position N8 seems to be extremely important, whilst 3-, 4- and 5-membered rings exhibit the best affinity for the target kinases.
Concerning the activity against CLK1, comparison of the results obtained for compounds 12a and 12f (IC50 = 0.031 μM and 0.091 μM, respectively) with those obtained for 13a and 13f (IC50 = 0.06 μM and 0.071 μM, respectively), demonstrates that the presence of methyl or benzyl carbimidates at C2 may have a slightly beneficial effect on the inhibitory activity, accompanied by the same cyclopropyl substituent on N8. The same phenomenon is observed when data concerning DYRK1A and GSK-3α/β kinases are compared. The DYRK1A-IC50 values obtained for 12a, 12d–f and 13a, 13d–f series are mainly in the submicromolar range (0.13 μM < IC50 < 0.39 μM) except for compounds 12a and 13a (IC50 = 0.091 μM and 0.06 μM, respectively), 12e and 13e (IC50 = 0.072 μM and 0.062 μM, respectively) and 13f (IC50 = 0.00.59 μM) for which nanomolar IC50 values were observed. These five products show interesting activity against DYRK1A. As described above for CLK1, the interesting results concern the fact that two different carbimidate groups gave similar affinity for the same kinase despite changes in the size of their substituents in position N8. This may suggest the existence of a spatial zone in the front pocket of the active site of the kinases which is relatively tolerant to various sizes of the molecules with 3-, 4- or 5- membered cyclolalkyl groups.
In the case of GSK-3α/β, the nanomolar values obtained for compounds 12a, 12d–h and 13a, 13d–h are spectacular and the list of compounds able to show very good affinity for the target kinase was extended to 12d–13d (IC50 = 0.041 μM and 0.030 μM, respectively) and 12g–13g (IC50 = 0.030 μM and 0.083 μM, respectively). For GSK-3α/β in particular, the fact that its hydrophobic back-pocket was recognized to be larger than in the case of the two other kinases (CLK1 and DYRK1A) may explain why 12d and 12g show very good affinity for this kinase. It may be suggested that the space available in this back-pocket allowed the molecules to shift and to have a better interaction with GSK-3α/β than in the other enzymes in which hydrophobic back-pocket are known to have a reduced size. In the same time the displacement of the tricyclic core into the site may allow the front pocket to be more tolerant and accept other substituents in N8.
All these results demonstrate that it is difficult to define any role for the various substituents located at position 8 of the thiazolo[5,4-f]quinazolin-9(8H)-one. The presence of small-sized cycloalkyl groups linked to the front pocket is favorable to the development of further SAR studies, whilst the other side of the molecule (carbimidate function) will perhaps serve to discriminate the pertinent kinases such as CLK1, DYRK1A and GSK-3α/β (Scheme 5). Although this needs to be confirmed, the results described in this study indicate clearly that the carbimidate function remains crucial for the multi-target inhibitory activity of kinases with such heterocyclic structures [13,14,15].
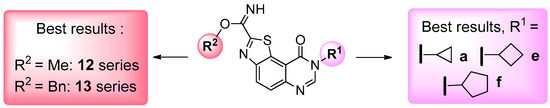
Scheme 5.
Structures of the lead compounds (nanomolar IC50 values) identified in this study.
The first kinase CLK1 is one of the four isoforms (CLK1-4) of the cdc2-like kinase family. In humans, the highest levels of CLK1 expression were found in the brain. It was described that CLK inhibitors may alter the splicing of microtubule-associated protein tau implicated in AD and Parkinson’s disease [39]. The second kinase targeted by the compounds described in this study is DYRK1A. Evidence for the role of overexpressed DYRK1A in various neurodegenerative diseases and Down syndrome is now well established and it has become an attractive drug target for numerous research groups [40,41,42]. The third kinase inhibited by the lead molecules in this study was GSK-3α/β which has definitely gained the attention of research groups and industry. This very versatile kinase has become a key target in type II diabetes, bipolar disorder, cancer, chronic inflammatory and immune disorder and, more importantly, in neurodegenerative diseases, making this enzyme a main target for AD [43].
Because drugs focusing their activity against a single target may generate low benefits, new studies are encouraged in the direction of multi-targeting strategies. Therefore, developing molecules showing submicromolar affinities on a panel of three kinases seems to be of great interest [17]. In this sense the new thiazolo[5,4-f]quinazolin-9(8H)-one series described in this paper need to be developed for the discovery of valuable and pertinent multi-kinases inhibitors.
3. Materials and Methods
3.1. General Information
Starting materials were obtained commercially and used without further purification. All reactions were monitored by thin-layer chromatography with silica gel 60 F254 precoated aluminium plates (0.25 mm). Visualization was performed with a UV light at wavelengths of 254 and 312 nm. Purifications were conducted with a flash column chromatography system equipped with a dual UV/Vis spectrophotometer (200–600 nm), a fraction collector (176 tubes), a dual piston pump (1 to 200 mL/min, Pmax = 15 bar), which allowed quaternary gradients, and an additional inlet for air purge. Melting points of solid compounds were measured with a SMP3 Melting Point instrument (STUART, Bibby Scientific Ltd, Roissy, France) with a precision of ±1.5 °C. IR spectra were recorded with a Spectrum 100 Series FTIR spectrometer (PerkinElmer, Villebon S/Yvette, France). Liquids and solids were investigated with a single-reflection attenuated total reflectance (ATR) accessory; the absorption bands are given in cm−1. NMR spectra (1H and 13C) were acquired at 295 K using a WP 300 spectrometer an AVANCE 300 MHz spectrometer (Bruker, Wissembourg, France) at 300 and 75.4 MHz, using TMS as an internal standard. Coupling constants J are in Hz, and chemical shifts are given in ppm. Signals in 13C spectra were assigned based on the result of 13C DEPT135 experiments (see Supplementary Materials). Mass spectrometry was performed by the Mass Spectrometry Laboratory of the University of Rouen. The mass spectra [ESI, EI, and field desorption (FD)] were recorded with a LCP 1er XR spectrometer (WATERS, Guyancourt, France). Microwave experiments were conducted in a commercial microwave reactor especially designed for synthetic chemistry. Start STM (Milestone S.r.l., Bergamo, Italy) is a multi-mode cavity with a microwave power delivery system ranging from 0 to 1200 W. The temperatures of the reactions were mainly monitored via contact-less infrared pyrometer which was calibrated in control experiments with a fibre-optic contact thermometer protected in a Teflon coated ceramic well inserted directly in the reaction mixture. Open vessel experiments were carried out in a 50–250 mL round bottom flask fitted with a reflux condenser. The vessel contents were stirred by means of an adjustable rotating magnetic plate located below the floor of the microwave cavity and a Teflon-coated magnetic stir bar inside the vessel. Temperature and power profiles were monitored in both cases through the EASY-Control software provided by the manufacturer (Milestone S.r.l., Bergamo, Italy). The times indicated in the various protocols are the times measured when the mixtures reached the programmed temperature after a ramp period of 2 min.
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of Carbonitriles 11a–n from 10
(E)-Methyl 2-cyano-6-([(dimethylamino)methylene]amino)benzo[d]thiazole-7-carboxylate (10): To a stirred solution of methyl 6-amino-2-cyanobenzo[d]thiazole-7-carboxylate (1, 1.00 g, 4.29 mmol) in methylene chloride (20 mL) was added Vilsmeier-Haack reagent (0.824 g, 6.44 mmol, 1.5 equiv) at room temperature. The resulting mixture was stirred at room temperature for 2 h. On completion, the crude mixture was diluted with methylene chloride (40 mL) and a saturated aqueous solution of NaHCO3 (40 mL). After 15 min of stirring, the product was extracted twice with methylene chloride. The combined organic layers were washed with brine and dried over MgSO4. Evaporation of solvent gave the amidine product 10 as a pale yellow solid (1.11 g, 90%), mp. 136–138 °C; 1H-NMR (DMSO-d6) δ 8.23 (d, J = 9.0 Hz, 1H, H4), 7.75 (s, 1H, CH(N)), 7.74 (d, J = 9.0 Hz, 1H, H5), 3.87 (s, 3H, OCH3), 3.10 (s, 3H, NCH3), 3.03 (s, 3H, NCH3); 13C-NMR (DMSO-d6) δ 167.1, 155.8, 154.8, 146.7, 138.3, 135.7, 128.7, 125.4, 114.8, 113.3, 52.3, 34.1; νmax 2952, 2224 (ν C≡N), 1622, 1569, 1423, 1331, 1258, 1098, 1016, 831 cm−1; HRMS calcd for C13H13N4O2S [M + H]+ 289.0759 found 289.0746.
In a sealed tube, a suspension of (E)-methyl 2-cyano-6-([(dimethylamino)methylene]-amino)benzo[d]thiazole-7-carboxylate (10, 125 mg, 0.43 mmol) and the appropriate amine (0.45 mmol, 1.05 equiv) in acetic acid (600 μL) was irradiated under microwaves at 100 °C for 6–10 min. The solvent was removed under vacuum to provide a crude residue which was purified by flash chromatography on silica gel with methylene chloride/ethyl acetate (100:0 to 50:50, v/v) as eluent to furnish the expected 8-substituted thiazoloquinazolinone-2-carbonitrile derivatives.
8-Cyclopropyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11a): white solid (90.0 mg, 78%), mp. 248–250 °C; 1H-NMR (DMSO-d6) δ 8.63 (d, J = 8.7 Hz, 1H, H4), 8.61 (s, 1H, H2), 7.99 (d, J = 9.0 Hz, 1H, H5), 3.42–3.36 (m, 1H, NCH), 1.13–1.09 (m, 4H, CH); 13C-NMR (DMSO-d6) δ 160.2, 150.3, 149.5, 148.2, 139.2, 131.6, 129.9, 127.8, 115.4, 113.5, 29.7, 5.9 (2C); νmax 3067, 2233 (C≡N), 1664, 1579, 1441, 1353, 1303, 1222, 1038, 839, 692 cm−1; HRMS calcd for C13H9N4OS [M + H]+ 269.0497 found 269.0487.
8-(2-Methoxyethyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11b): pale beige solid (100.8 mg, 82%), mp. 204–206 °C; 1H-NMR (CDCl3) δ 8.67 (d, J = 9.0 Hz, 1H, H4), 8.62 (s, 1H, H2), 8.02 (d, J = 9.0 Hz, 1H, H5), 4.32 (t, J = 5.1 Hz, 2H, OCH2), 3.69 (t, J = 5.1 Hz, 2H, NCH2), 3.26 (s, 3H, OCH3); 13C-NMR (DMSO-d6) δ 159.9, 150.3, 149.8, 148.7, 139.2, 131.7, 130.1, 127.9, 115.4, 113.5, 68.8, 58.1, 46.0; νmax 3098, 2932, 2891, 2237 (ν C≡N), 1656, 1582, 1352, 1111, 1013, 835, 559 cm−1; HRMS calcd for C13H11N4O2S [M + H]+ 287.0603 found 287.0605.
8-(3-Methoxypropyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11c): pale beige solid (103.4 mg, 80%), mp. 128–130 °C; 1H-NMR (DMSO-d6) δ 8.68 (s, 1H, H7), 8.65 (d, J = 9.0 Hz, 1H, H4), 8.01 (d, J = 9.0 Hz, 1H, H5), 4.19 (t, J = 6.9 Hz, 2H, OCH2), 3.41 (t, J = 6.0 Hz, 2H, NCH2), 3.21 (s, 3H, OMe), 2.01 (dt, J = 6.9, 6.0 Hz, 2H, CH2); 13C-NMR (DMSO-d6) δ 159.0, 150.3, 149.6, 148.7, 139.2, 131.7, 129.9, 127.9, 115.5, 113.6, 69.1, 57.9, 44.6, 28.2; νmax 3058, 2901, 2878, 2831, 2232 (ν C≡N), 1656, 1586, 1470, 1354, 1156, 1110, 889, 847 cm−1; HRMS calcd for C14H13N4O2S [M + H]+ 301.0759 found 301.075.
8-(3-Isopropyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11d): obtained in 58% yield as a white solid (mp > 265 °C). Data supporting its chemical structure are reported in [6].
8-Cyclopentyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11e): beige solid (89.2 mg, 70%), mp > 265°C; 1H-NMR (DMSO-d6) δ 8.75 (s, 1H, H2), 8.66 (d, J = 8.7 Hz, 1H, H4), 8.02 (d, J = 8.7 Hz, 1H, H5), 5.13–5.06 (m, 1H, NCH), 2.22–1.61 (m, 8H, CH); 13C-NMR (CDCl3 + DMSO-d6) δ 159.0, 150.4, 147.3, 139.2, 129.8, 127.8, 113.7, 57.2, 30.0, 24.1; νmax 3074, 2961, 2233 (ν C≡N), 1657, 1583, 1473, 1356, 1254, 1105, 840 cm−1; HRMS calcd for C15H13N4OS [M + H]+ 297.0810 found 297.0820.
8-Cyclobutyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11f): white solid (88.5 mg, 73%), mp > 265 °C; 1H-NMR (CDCl3) δ 8.52 (d, J = 8.7 Hz, 1H, H4), 8.40 (s, 1H, H2), 7.98 (d, J = 8.7 Hz, 1H, H5), 5.21–5.09 (m, 1H, NCH), 2.71–2.61 (m, 2H, CH), 2.54–2.40 (m, 2H, CH), 2.07–2.02 (m, 2H, CH); 13C-NMR (DMSO-d6) δ 159.7, 151.6, 148.8, 145.2, 140.3, 132.4, 130.6, 128.2, 116.1, 113.4, 51.2, 30.0 (2C), 15.6; νmax 3441, 3296, 3092, 2233 (ν C≡N), 1662, 1586, 1280, 1145, 1059, 1114, 841 cm−1; HRMS calcd for C14H11N4OS [M + H]+ 283.0654 found 283.0660.
8-Cyclohexyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11g): white solid (92.4 mg, 69%), mp. 256–258 °C; 1H-NMR (DMSO-d6) δ 8.81 (s, 1H, H7), 8.65 (d, J = 9.0 Hz, 1H, H4), 8.01 (d, J = 9.0 Hz, 1H, H5), 4.78–4.71 (m, 1H, NCH), 2.0–1.21 (m, 10H, CH); 13C-NMR (DMSO-d6) δ 158.6, 150.3, 148.1, 147.2, 139.2, 131.9, 130.0, 127.9, 115.4, 113.6, 54.7, 31.1 (2C), 25.5 (2C), 24.6; νmax 3060, 2940, 2869, 2226 (ν C≡N), 1657, 1583, 1466, 1448, 1388, 1346, 1183, 1127, 830 cm−1; HRMS calcd for C16H15N4OS [M + H]+ 311.0967 found 311.0966.
8-Dimethylamino-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11h): beige solid (98.0 mg, 84%), mp > 265 °C; 1H-NMR (DMSO-d6) δ 8.68 (d, J = 8.8 Hz, 1H, H4), 8.61 (s, 1H, H2), 8.03 (d, J = 8.8 Hz, 1H, H5), 3.10 (s, 6H, CH3); 13C-NMR (DMSO-d6) δ 159.0, 150.9, 150.3, 148.1, 139.4, 131.5, 130.1, 128.0, 117.1, 113.5, 44.2 (2C); νmax 3080, 2966, 2887, 2231 (C≡N), 1659, 1577, 1442, 1349, 1299, 1156, 1060, 839, 696 cm−1; HRMS calcd for C12H10N5OS [M + H]+ 272.0606 found 272.0600.
8-(2-Dimethylaminoethyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11i): white solid (99.1 mg, 77%), mp > 265 °C; 1H-NMR (CDCl3) δ 8.52 (d, J = 9.0 Hz, 1H, H4), 8.30 (s, 1H, H7), 7.98 (d, J = 9.0 Hz, 1H, H5), 4.22 (t, J = 6.0 Hz, 2H, NCH2), 2.72 (t, J = 6.0 Hz, 2H, NCH2), 2.30 (s, 6H, NCH3); 13C-NMR (CDCl3) δ 159.5, 151.3, 149.0, 148.3, 140.0, 132.3, 130.3, 128.1, 116.2, 113.2, 57.7, 45.0; νmax 3072, 2949, 2826, 2781, 2235 (ν C≡N), 1667, 1585, 1465, 1354, 1151, 931, 832 cm−1; HRMS calcd for C14H14N5O2S [M + OH]− 316.0868 found 316.0872.
8-(2-Dimethylaminoethyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11j): yellow solid (114.5 mg, 78%), mp. 192–194 °C; 1H-NMR (CDCl3) δ 8.52 (d, J = 9.0 Hz, 1H, H4), 8.28 (s, 1H, H7), 7.97 (d, J = 9.0 Hz, 1H, H5), 4.23 (t, J = 5.7 Hz, 2H, NCH2), 3.68–3.65 (m, 4H, CH), 2.77 (t, J = 5.7 Hz, 2H, NCH2), 2.54–2.51 (m, 4H, CH); 13C-NMR (CDCl3) δ 159.7, 151.5, 149.2, 148.4, 140.3, 132.5, 130.6, 128.3, 116.3, 113.4, 67.1 (2C), 57.0, 53.9 (2C), 44.0; νmax 3060, 2925, 2815, 1659, 1586, 1452, 1345, 1112, 833 cm−1; HRMS calcd for C16H16N5O2S [M + H]+ 342.1025 found 342.1026.
8-(p-Tolyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11k): white solid (116.4 mg, 85%), mp > 265 °C; 1H-NMR (DMSO-d6) δ 8.72 (d, J = 9.0 Hz, 1H, H4), 8.67 (s, 1H, H2), 8.09 (d, J = 9.0 Hz, 1H, H5), 7.51 (d, J = 8.4 Hz, 2H, HAr), 7.42 (d, J = 8.4 Hz, 2H, HAr), 2.43 (s, 3H, CH3); νmax 3057, 2225 (ν C≡N), 1668, 1580, 1490, 1353, 1273, 1189, 1091, 847, 835, 802, 576 cm−1.
8-(4-Methoxyphenyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11l): white solid (33.7 mg, 46%), mp > 265 °C; 1H-NMR (DMSO-d6) δ 8.71 (d, J = 8.7 Hz, 1H, H4), 8.66 (s, 1H, H2), 8.08 (d, J = 8.7 Hz, 1H, H5), 7.56 (d, J = 9.0 Hz, 2H, ArH), 7.15 (d, J = 9.0 Hz, 2H, ArH), 3.56 (s, 3H, OCH3); νmax 3099, 2932, 2237 (ν C≡N), 1656, 1584, 1352, 1111, 1013, 835 cm−1; HRMS calcd for C17H11N4O3S [M+OH]− 351.0552 found 351.0548.
8-(4-Dimethylaminophenyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11m): yellow solid (121.0 mg, 81%), mp. 224–226 °C; 1H-NMR (DMSO-d6) δ 8.70 (d, J = 9.0 Hz, 1H, H4), 8.63 (s, 1H, H2), 8.07 (d, J = 9.0 Hz, 1H, H5), 7.40 (d, J = 9.0 Hz, 2H, ArH), 6.87 (d, J = 9.0 Hz, 2H, ArH), 2.99 (s, 6H, NCH3); νmax 3374, 3061, 2234 (ν C≡N), 1661, 1611, 14583, 1518, 1353, 1277, 1189, 836, 805 cm−1; HRMS calcd for C18H14N5O2S [M + OH]− 364.0868 found 364.0872.
8-Methyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbonitrile (11n): white solid (87.5 mg, 84%), mp. 248–250 °C; 1H-NMR (CDCl3) δ 8.53 (d, J = 9.0 Hz, 1H, H4), 8.30 (s, 1H, H2), 7.98 (d, J = 9.0 Hz, 1H, H5), 3.77 (s, 3H, NCH3); 13C-NMR (CDCl3) δ 160.0, 151.5, 149.2, 147.9, 140.3, 132.2, 130.5, 128.2, 116.2, 113.3, 34.5; νmax 2987, 2904, 2234 (ν C≡N), 1664, 1669, 1588, 1357, 1334, 1055, 841 cm−1; HRMS calcd for C11H7N4OS [M + H]+ 243.0341 found 243.0334.
3.2.2. General Procedure for the Synthesis of Carbimidates 12a–n, 13a–h, 14a–e and 15a–c from 11a–n
To a stirred solution of 11a–n (0.25 mmol, 1 equiv) in appropriate alcohol (2.5 mL) was added an aqueous solution of sodium hydroxyde (2.5 M, 100 μL, 1.0 equiv) and the resulting mixture was stirred at room temperature for 1 h under argon atmosphere. The solvent was removed under vacuum and the crude residue was adsorbed on Celite® and purified by flash chromatography on silica gel with methylene chloride/methanol (100:0 to 2:98; v/v) as eluent to furnish the desired carbimidate compound.
Ethyl 8-cyclopropyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidates 12a–n
Methyl 8-cyclopropyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12a): white solid (68 mg, 91%), mp. 234–236 °C; 1H-NMR (CDCl3) δ 8.94 (s, 1H, NH), 8.40 (d, J = 9.0 Hz, 1H, H4), 8.22 (s, 1H, H7), 7.78 (d, J = 9.0 Hz, 1H, H5), 4.04 (s, 3H, OCH3), 3.37–3.29 (m, 1H, NCH), 1.27–1.23 (m, 2H, CH), 1.01–1.00 (m, 2H, CH); 13C-NMR (CDCl3) δ 161.9 (2C), 160.9, 151.8, 147.2, 147.0, 133.0, 129.8, 126.7, 116.3, 54.4, 29.7, 6.6 (2C); νmax 3272, 2918, 2848, 1728, 1664, 1583, 1498, 1438, 1339, 1141, 1060, 893, 837 cm−1; HRMS calcd for C14H13N4O2S [M + H]+ 301.0759 found 301.0771.
Methyl 8-(2-methoxyethyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12b): white solid (56.5 mg, 71%), mp. 196–198 °C; 1H-NMR (DMSO-d6) δ 9.46 (s, 1H, NH), 8.57 (d, J = 8.7 Hz, 1H, H4), 8.54 (s, 1H, H7), 7.93 (d, J = 8.7 Hz, 1H, H5), 4.31 (t, J = 5.1 Hz, 2H, OCH2), 3.98 (s, 1H, OCH3), 3.68 (t, J = 5.1 Hz, 2H, NCH2), 3.26 (s, 3H, OCH3); 13C-NMR (DMSO-d6) δ 160.6, 159.9, 159.0, 150.9, 149.0, 147.6, 131.7, 129.6, 126.9, 115.9, 68.9, 58.1, 54.1, 45.9; νmax 3271, 2914, 1651, 1587, 1444, 1340, 1111, 1065 cm−1; HRMS calcd for C14H15N4O2S [M + H]+ 319.0865 found 319.0880.
Methyl 8-(3-methoxypropyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12c): white solid (63.0 mg, 76%), mp. 168–170 °C; 1H-NMR (DMSO-d6) δ 9.45 (s, 1H, NH), 8.59 (s, 1H, H7), 8.54 (d, J = 8.7 Hz, 1H, H4), 7.91 (d, J = 8.7 Hz, 1H, H5), 4.16 (t, J = 6.2 Hz, 2H, NCH2), 3.98 (s, 3H, OCH3), 3.40 (t, J = 5.7 Hz, 2H, OCH2), 3.22 (s, 3H, OCH3), 2.00 (dt, J = 6.2, 5.7 Hz, 2H, CH2); 13C-NMR (DMSO-d6) δ 160.6, 160.0, 159.0, 150.8, 148.7, 147.6, 131.6, 129.4, 126.8, 115.9, 69.1, 57.9, 54.0, 44.4, 28.2; νmax 3272, 2946, 1658, 1635, 1588, 1350, 1105, 889, 831 cm−1; HRMS calcd for C15H17N4O3S [M + H]+ 333.1021 found 333.1030.
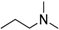
Methyl 8-isopropyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12d): white solid (69.5 mg, 92%), mp. 234–236 °C; 1H-NMR (DMSO-d6) δ 9.45 (s, 1H, NH), 8.73 (s, 1H, H7), 8.55 (d, J = 9.0 Hz, 1H, H4), 7.93 (d, J = 9.0 Hz, 1H, H5), 5.15–5.08 (sept, J = 6.9 Hz, 1H, NCH), 3.99 (s, 3H, OCH3), 1.50 (d, J = 6.9 Hz, 6H, CH3); 13C-NMR (DMSO-d6) δ 160.6, 160.0, 158.7, 150.8, 147.1, 146.1, 131.9, 129.5, 126.8, 115.8, 54.0, 47.0, 21.2 (2C); νmax 3315, 3061, 2977, 1673, 1657, 1583, 1347, 1072, 838, 699 cm−1; HRMS calcd for C14H15N4O2S [M + H]+ 303.0916 found 303.0919.
Methyl 8-cyclopentyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12e): white solid (73 mg, 89%), mp. 226–228 °C; 1H-NMR (DMSO-d6) δ 9.45 (s, 1H, NH), 8.66 (s, 1H, H7), 8.55 (d, J = 8.7 Hz, 1H, H4), 7.91 (d, J = 8.7 Hz, 1H, H5), 5.13–5.05 (m, 1H, NCH), 3.98 (s, 3H, OCH3), 2.22–2.09 (m, 2H, CH), 2.04–1.87 (m, 4H, CH), 1.77–1.65 (m, 2H, CH); 13C-NMR (DMSO-d6) δ 160.7, 160.0, 159.1, 150.9, 147.1, 146.6, 131.9, 129.5, 126.8, 115.8, 56.6, 54.1, 31.0 (2C), 24.1 (2C); νmax 3293, 2933, 2862, 1657, 1636, 1579, 1494, 1434, 1349, 1254, 840 cm−1; HRMS calcd for C16H17N4O2S [M + H]+ 329.1072 found 329.1077.
Methyl 8-cyclobutyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12f): white solid (59.7 mg, 76%), mp. 260–262 °C; 1H-NMR (DMSO-d6) δ 9.46 (s, 1H, NH), 8.72 (s, 1H, H7), 8.56 (d, J = 8.7 Hz, 1H, H4), 7.93 (d, J = 8.7 Hz, 1H, H5), 5.15–5.07 (m, 1H, NCH), 3.99 (s, 3H, OCH3), 1.90–1.85 (m, 4H, CH), 1.23–1.18 (m, 2H, CH); 13C-NMR (DMSO-d6) δ 162.0 (2C), 159.8, 151.9, 147.5, 144.3, 133.2, 129.9, 126.8, 116.5, 54.5, 50.9, 29.9 (2C), 15.5; νmax 3296, 2983, 2955, 1661, 1636, 1580, 1494, 1436, 1351, 1255, 1155, 841 cm−1; HRMS calcd for C15H15N4O2S [M + H]+ 315.0916 found 315.0918.
Methyl 8-cyclohexyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12g): white powder (78 mg, 92%), mp. 246–248 °C; 1H-NMR (DMSO-d6) δ 9.45 (s, 1H, NH), 8.73 (s, 1H, H7), 8.56 (d, J = 8.7 Hz, 1H, H4), 7.93 (d, J = 8.7 Hz, 1H, H5), 4.79–4.72 (m, 1H, NCH), 3.99 (s, 3H, OCH3), 1.97–1.38 (m, 10, CH); 13C-NMR (DMSO-d6) δ 162.0, 161.9, 159.5, 151.8, 147.3, 144.2, 133.4, 129.9, 126.7, 116.6, 54.5, 32.7 (2C), 26.0 (2C), 35.3; νmax 3298, 2946, 2856, 1658, 1586, 1333, 1139, 1078, 834 cm−1; HRMS calcd for C17H19N4O2S [M + H]+ 343.1229 found 343.1224.
Methyl 8-dimethylamino-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12h): white solid (53.1 mg, 70%), mp. 246–248 °C; 1H-NMR (DMSO-d6) δ 9.46 (s, 1H, NH), 8.57 (d, J = 9.0 Hz, 1H, H4), 8.52 (s, 1H, H7), 7.94 (d, J = 9.0 Hz, 1H, H5), 3.99 (s, 3H, OCH3), 3.10 (s, 6H, NCH3); 13C-NMR (CDCl3) δ 162.1, 162.0, 160.0, 151.9, 149.3, 147.4, 133.0, 130.0, 127.0, 118.1, 54.5, 45.0 (2C); νmax 3299, 3073, 3036, 2945, 1670, 1637, 1580, 1437, 1347, 1158, 1079, 838, 808, 706 cm−1; HRMS calcd for C13H14N5O2S [M + H]+ 304.0868 found 304.0871.
Methyl 8-[(3-dimethylamino)ethyl]-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12i): white solid (66.3 mg, 80%), mp. 178–180 °C; 1H-NMR (DMSO-d6) δ 9.46 (s, 1H, NH), 8.57 (s, 1H, H7), 8.56 (d, J = 9.0 Hz, 1H, H4), 7.92 (d, J = 9.0 Hz, 1H, H5), 4.21 (t, J = 6.0 Hz, 2H, NCH2), 3.98 (s, 3H, OCH3), 2.61 (t, J = 6.0 Hz, 2H, NCH2), 2.20 (s, 6H, NCH3); 13C-NMR (DMSO-d6) δ 158.9, 150.3, 149.7, 148.7, 139.1, 131.7, 130.0, 127.9, 115.4, 113.5, 57.0, 45.2 (2C), 44.0, 30.7; νmax 3201, 2981, 2774, 1650, 1587, 1442, 1341, 1066, 826 cm−1; HRMS calcd for C15H18N5O2S [M + H]+ 332.1181 found 332.1178.
Methyl 8-(2-morpholinoethyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12j): light yellow solid (80.3 mg, 86%), mp. 190–192 °C; 1H-NMR (DMSO-d6) δ 9.41 (s, 1H, NH), 8.56 (s, 1H, H7), 8.52 (d, J = 9.0 Hz, 1H, H4), 7.92 (d, J = 9.0 Hz, 1H, H5), 4.22 (t, J = 6.0 Hz, 2H, NCH2), 3.98 (s, 3H, OCH3), 3.54–3.51 (m, 4H, OCH2), 2.67 (t, J = 6.0 Hz, 2H, NCH2), 2.48–2.46 (m, 4H, NCH2); 13C-NMR (DMSO-d6) δ 160.6, 160.0, 159.0, 150.8, 148.9, 147.6, 131.7, 129.5, 126.8, 115.8, 115.8, 66.2 (2C), 56.2, 54.0, 53.1 (2C), 42.9; νmax 3452, 3296, 2944, 2804, 1649, 1587, 1497, 1439, 1340, 1065, 1112, 1065, 825 cm−1; HRMS calcd for C17H20N5O3S [M + H]+ 374.1287 found 374.1278.
Methyl 8-(p-tolyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12k): white solid (59.0 mg, 68%), mp > 265 °C; 1H-NMR (DMSO-d6) δ 9.47 (s, 1H, NH), 8.55 (d, J = 8.7 Hz, 1H, H5), 8.57 (s, 1H, H7), 7.99 (d, J = 8.7 Hz, 1H, H4), 7.51 (d, J = 8.4 Hz, 2H, HAr), 7.42 (d, J = 8.4 Hz, 2H, HAr), 3.97 (s, 3H, OCH3), 2.42 (s, 3H, CH3); νmax 3257, 3060, 1644, 1576, 1490, 1320, 1263, 1185.0, 803, 781 cm−1; HRMS calcd for C18H15N4O2S [M + H]+ 351,0916 found 351.0950.
Methyl 8-(4-methoxyphenyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12l): white solid (74.6 mg, 82%), mp > 265 °C; 1H-NMR (DMSO-d6) δ 9.47 (s, 1H, NH), 8.61 (d, J = 8.7 Hz, 1H, H4), 8.57 (s, 1H, H7), 7.99 (d, J = 8.7 Hz, 1H, H5), 7.55 (d, J = 9.0 Hz, 2H, HAr), 7.15 (d, J = 9.0 Hz, 2H, ArH), 3.98 (s, 3H, OCH3), 3.86 (s, 3H, OCH3); νmax 3307, 3061, 1671, 1582, 1516, 1339, 1261, 832 cm−1; HRMS calcd for C18H15N4O3S [M + H]+ 367.0865 found 367.0873.
Methyl 8-(4-dimethylaminophenyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12m): yellow solid (70.2 mg, 74%), mp > 265 °C; 1H-NMR (DMSO-d6) δ 9.46 (s, 1H, NH), 8.60 (d, J = 8.7 Hz, 1H, H4), 8.54 (s, 1H, H7), 7.98 (d, J = 8.7 Hz, 1H, H5), 7.38 (d, J = 9.0 Hz, 2H, HAr), 6.86 (d, J = 9.0 Hz, 2H, HAr), 3.98 (s, 3H, OCH3), 2.99 (s, 6H, NCH3); νmax 3245, 2932, 2854, 1659, 1587, 1347, 1192, 836, 805 cm−1; HRMS calcd for C19H18N5O2S [M + H]+ 380.1181 found 380.1196.
Methyl 8-methyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (12n): white solid (61.7 mg, 90%), mp. 244–246 °C; 1H-NMR (DMSO-d6) δ 9.44 (s, 1H, NH), 8.62 (s, 1H, H7), 8.54 (d, J = 8.7 Hz, 1H, H4), 7.91 (d, J = 8.7 Hz, 1H, H5), 3.98 (s, 3H, OCH3), 3.32 (s, 3H, NCH3); 13C-NMR (DMSO-d6) δ 160.6, 160.0, 159.5, 150.8, 149.1, 147.8, 135.4, 131.6, 129.4, 126.9, 115.8, 54.0, 33.9; νmax 3257, 2949, 1655, 1589, 1436, 1339, 1153, 1064, 839, 716, 498 cm−1; HRMS calcd for C12H11N4O2S [M + H]+ 275.0603 found 275.0608.
Benzyl 8-cyclopropyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidates (13a–h)
Benzyl 8-cyclopropyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (13a): white solid (48 mg, 85%), mp. 214–216 °C; 1H-NMR (CDCl3) δ 9.60 (s, 1H, NH), 8.56 (d, J = 9.0 Hz, 1H, H4), 8.53 (s, 1H, H7), 7.92 (d, J = 9.0 Hz, 1H, H5), 7.57–7.40 (m, 5H, Ph), 5.48 (s, 2H, CH2Ph), 3.30–3.15 (m, 1H, CH), 1.11–1.06 (m, 4H, CH); 13C-NMR (CDCl3) δ 162.1, 161.2, 161.1, 152.0, 147.4, 147.1, 136.0, 133.3, 130.0, 128.7 (2C), 128.3, 127.9 (2C), 126.9, 116.5, 68.8, 29.8, 6.7 (2C); νmax 3286, 3024, 1660, 1636, 1586, 1491, 1335, 1155, 1084, 833, 734 cm−1; HRMS calcd for C20H17N4O2S [M + H]+ 377.1072 found 377.1082.
Benzyl 8-(2-methoxyethyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (13b): white solid (57.1 mg, 58%), mp. 160–162°C; 1H-NMR (DMSO-d6) δ 9.61 (s, 1H, NH), 8.58 (d, J = 9.0 Hz, 1H, H4), 8.54 (s, 1H, H7), 7.93 (d, J = 9.0 Hz, 1H, H5), 7.56–7.38 (m, 5H, Ph), 5.47 (s, 2H, CH2Ph), 4.29 (t, J = 5.1 Hz, 2H, OCH2), 3.67 (t, J = 5.1 Hz, 2H, NCH2), 3.26 (s, 3H, OCH3); 13C-NMR (DMSO-d6) δ 160.7, 159.1, 159.0, 151.0, 149.0, 147.6, 136.3 ,131.7, 129.7, 128.5 (2C), 128.0, 127.8 (2C), 126.9, 115.9, 69.1, 67.8, 58.1, 45.9; νmax 3456, 3396, 3275, 2925, 1656, 1589, 1334, 1105, 1005, 837 cm−1; HRMS calcd for C20H19N4O3S [M + H]+ 395.1178 found 395.1177.
Benzyl 8-(3-methoxypropyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (13c): white solid (56.2 mg, 55%), mp. 168–170 °C; 1H-NMR (DMSO-d6) δ 9.60 (s, 1H, NH), 8.59 (s, 1H, H7), 8.57 (d, J = 9.0 Hz, 1H, H4), 7.93 (d, J = 9.0 Hz, 1H, H5), 7.56–7.39 (m, 5H, Ph), 5.47 (s, 1H, CH2Ph), 4.16 (t, J = 6.9 Hz, 2H, NCH2), 3.40 (t, J = 6.0 Hz, 2H, OCH2), 3.21 (s, 3H, OCH3), 2.00 (dt, J = 6.9, 6.0 Hz, 2H, CH2); 13C-NMR (DMSO-d6) δ 160.7, 159.2, 159.1, 150.9, 148.7, 147.7, 136.3 ,131.7, 129.5, 128.4 (2C), 128.0, 127.8 (2C), 126.9, 116.0, 69.1, 67.8, 57.9, 44.4, 28.2; νmax 3272, 3078, 2923, 2865, 1666, 1647, 1584, 1453, 1317, 1113, 1050, 890, 837, 749 cm−1; HRMS calcd for C21H21N4O3S [M + H]+ 409.1334 found 409.1333.
Benzyl 8-isopropyl-9-oxo-8,9-dihydro[1,3]thiazolo[5,4-f]quinazoline-2-carbimidate (13d): white solid (61 mg, 65%), mp. 212–214 °C; 1H-NMR (DMSO-d6) δ 9.60 (s, 1H, NH), 8.72 (s, 1H, H7), 8.56 (d, J = 8.7 Hz, 1H, H4), 7.93 (d, J = 8.7 Hz, 1H, H5), 7.56–7.38 (m, 5H, Ph), 5.47 (s, 2H, CH2Ph), 5.02–5.11 (sept, J = 6.9 Hz, 1H, NCH), 1.50 (d, J = 6.9 Hz, 6H, CH3). Other data supporting its chemical structure are reported in [6].
Benzyl 8-cyclopentyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (13e): white solid (91 mg, 90%), mp. 214–216 °C; 1H-NMR (DMSO-d6) δ 9.58 (s, 1H, NH), 8.65 (s, 1H, H7), 8.55 (d, J = 8.7 Hz, 1H, H4), 7.92 (d, J = 8.7 Hz, 1H, H5), 7.57–7.39 (m, 5H, Ph), 5.47 (s, 2H, CH2Ph), 5.13–5.02 (m, 1H, NCH), 2.21–2.09 (m, 2H, CH2), 2.00–1.87 (m, 4H, 2 × CH2), 1.79–1.60 (m, 2H, CH2); 13C-NMR (DMSO-d6) δ 161.9, 161.3, 160.0, 151.9, 147.4, 144.7, 136.0, 133.4, 129.9, 128.7 (2C), 128.3, 128.0 (2C), 126.8, 116.6, 68.8, 57.0, 32.2 (2C), 24.6 (2C); νmax 3300, 2926, 2869, 1671, 1660, 1632, 1583, 1491, 1335, 1318, 1257, 1144, 830, 745 cm−1; HRMS calcd for C22H21N4O2S [M + H]+ 405.1385 found 405.1394.
Benzyl 8-cyclobutyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (13f): white solid (84 mg, 86%), mp. 234–236 °C; 1H-NMR (CDCl3) δ 9.11 (s, 1H, NH), 8.44 (d, J = 8.7 Hz, 1H, H4), 8.32 (s, 1H, H7), 7.89 (d, J = 8.7 Hz, 1H, H5), 7.53–7.36 (m, 5H, Ph), 5.50 (s, 2H, CH2Ph), 5.16–5.04 (m, 1H, NCH), 2.63 (m, 2H, 2 × CH), 2.52–2.43 (m, 2H,2 × CH), 2.02–2.00 (m, 2H, 2 x CH); 13C-NMR (CDCl3) δ 161.9, 161.3, 159.9, 152.0, 147.6, 144.3, 136.0, 133.3, 130.0, 128.7 (2C), 128.3, 128.0 (2C), 126.9, 116.5, 68.9, 51.2, 29.8 (2C), 15.6; νmax 3301, 3055, 2983, 2955, 2921, 1659, 1640, 1581, 1340, 1159, 830, 733 cm−1; HRMS calcd for C21H19N4O2S [M + H]+ 391.1229 found 391.1216.
Benzyl 8-cyclohexyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (13g): white solid (59 mg, 94%), mp. 212–214 °C; 1H-NMR (CDCl3) δ 9.11 (s, 1H, NH), 8.42 (d, J = 8.7 Hz, 1H, H4), 8.27 (s, 1H, H7), 7.85 (d, J = 8.7 Hz, 1H, H5), 7.52–7.34 (m, 5H, Ph), 5.50 (s, 2H, CH2Ph), 4.89–4.81 (m, 1H, NCH), 2.08-1.25 (m, 10H, 5 × CH2); 13C-NMR (CDCl3) δ 162.0, 161.4, 159.7, 152.0, 147.4, 144.3, 136.2, 133.6, 130.0, 128.8, 128.3, 127.9, 126.9, 68.9, 54.9, 32.8, 26.1, 25.4; νmax 3301, 2931, 2861, 1656, 1627, 1583, 1490, 1334, 1146, 1078, 833, 744, 700 cm−1; HRMS calcd for C23H23N4O2S [M + H]+ 419.1542 found 419.1546.
Benzyl 8-dimethylamino-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (13h): white solid (45 mg, 80%), mp. 190–192 °C; 1H-NMR (DMSO-d6) δ 9.61 (s, 1H, NH), 8.58 (d, J = 9.0 Hz, 1H, H4), 8.52 (s, 1H, H7), 7.94 (d, J = 9.0 Hz, 1H, H5), 7.57–7.37 (m, 5H, Ph), 5.48 (s, 2H, CH2Ph), 3.09 (s, 6H, 2 × CH3); 13C-NMR (DMSO-d6) δ 161.1, 159.1, 151.0, 150.0, 147.0, 138.3, 129.7, 128.5 (2C), 128.1, 127.9 (2C), 117.6, 67.9, 44.2 (2C); νmax 3291, 3038, 2922, 1688, 1642, 1578, 1493, 1444, 1330, 1295, 1164, 1072, 916, 827, 752, 699 cm−1; HRMS calcd for C19H18N5O2S [M + H]+ 380.1181 found 380.1183.
Ethyl 9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidates (14a–e)
Ethyl 8-cyclopropyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (14a): white solid (73.0 mg, 93%), mp. 248–250 °C; 1H-NMR (CDCl3) δ 8.94 (s, 1H, NH), 8.42 (d, J = 9.0. Hz, 1H, H4), 8.27 (s, 1H, H7), 7.86 (d, J = 9.0 Hz, 1H, H5), 4.49 (q, J = 7.2 Hz, 2H, OCH2), 3.41–3.33 (m, 1H, NCH), 1.48 (t, J = 7.2 Hz, 3H, CH3), 1.33–1.24 (m, 2H, CH), 1.06–1.03 (m, 2H, CH); 13C-NMR (CDCl3) δ 162.6, 161.4, 161.1, 152.0, 147.3, 147.1, 133.2, 130.0, 126.8, 116.5, 63.2, 29.7, 14.3, 6.7 (2C); νmax 3279, 2982, 1666, 1633, 1584, 1497, 1345, 1274, 1076, 866, 845 cm−1; HRMS calcd for C15H15N4O2S [M + H]+ 315.0916 found 315.0901.
Ethyl 8-(2-methoxyethyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carboximidoate (14b): white solid (63.2 mg, 76%), mp. 166–168 °C, 1H-NMR (DMSO-d6) δ 9.36 (br s, 1H, NH), 8.54 (d, J = 9.0 Hz, 1H, H4), 8.53 (s, 1H, H7), 7.90 (d, J = 9.0 Hz, 1H, H5), 4.42 (q, J = 7.2 Hz, 2H, OCH2), 4.30 (t, J = 5.1 Hz, 2H, OCH2), 3.68 (t, J = 5.1 Hz, 2H, NCH2), 3.27 (s, 3H, OCH3), 1.41 (t, J = 7.2 Hz, 3H, CH3); νmax 3308, 3160, 2985, 1663, 1589, 1347, 1112, 1005, 837 cm−1; HRMS calcd for C15H17N4O3S [M + H]+ 333.1021 found 333.1023.
Ethyl 8-(3-methoxypropyl)-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carboximidoate (14c): white solid (62 mg, 72%), mp. 172–174 °C; 1H-NMR (DMSO-d6) δ 9.37 (s, 1H, NH), 8.59 (s, 1H, H7), 8.55 (d, J = 8.9 Hz, 1H, H4), 7.91 (d, J = 8.9 Hz, 1H, H5), 4.42 (q, J = 7.2 Hz, 1H, CH2), 4.17 (t, J = 6.9 Hz, 2H, OCH2), 3.40 (t, J = 6.0 Hz, 2H, NCH2), 3.32 (s, 3H, OCH3), 2.00 (dt, J = 6.9, 6.0 Hz, 2H, CH2), 1.41 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (DMSO-d6) δ 160.9, 159.4, 159.1, 150.9, 148.7, 147.6, 131.7, 129.5, 126.8, 116.0, 69.1, 62.5, 57.9, 44.4, 28.2, 14.0; νmax 3280, 2973, 2867, 1660, 1640, 1581, 1327, 1107, 893, 827 cm−1; HRMS calcd for C16H19N4O3S [M + H]+ 347.1173, found 347.1178.
Ethyl 8-isopropyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carboximidoate (14d): white solid (63.3.mg, 80%), mp. 196–198 °C; 1H-NMR (DMSO-d6) δ 9.37 (s, 1H, NH), 8.72 (s, 1H, H7), 8.55 (d, 8.7 Hz, 1H, H4), 7.92 (d, 8.7 Hz, 1H, H5), 5.13–5.04 (m, 1H, NCH), 4.42 (q, J = 6.9 Hz, 2H, OCH2), 1.50 (d, J = 6.9 Hz, 6H, 2x CH3), 1.42 (t, 7.2 Hz, 1H, CH3). Other data supporting its chemical structure are reported in [6].
Ethyl 8-cyclopentyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (14e): white solid (79 mg, 92%), mp. 218-220 °C; 1H-NMR (CDCl3) δ 8.94 (s, 1H, NH), 8.41 (d, J = 9.0 Hz, 1H, H4), 8.27 (s, 1H, H7), 7.86 (d, J = 9.0 Hz, 1H, H5), 5.22 (m, 1H, NCH), 4.49 (q, J = 6.9 Hz, 2H, OCH2), 2.27 (m, 2H, CH), 1.95 (m, 6H, CH), 1.48 (t, J = 6.9 Hz, 3H, CH3); 13C-NMR (CDCl3) δ 162.3, 161.4, 159.8, 151.8, 147.2, 144.6, 133.2, 129.8, 126.6, 116.5, 63.1, 56.9, 32.1 (2C), 24.6 (2C), 14.2; νmax 3274, 3063, 2922, 1647, 1587, 1495, 1323, 21143, 1118, 1055, 836 cm−1; HRMS calcd for C17H19N4O2S [M + H]+ 343.1229 found 343.1232.
Isopropyl 9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidates 15a–c
Isopropyl 8-cyclopropyl-9-oxo-8,9-dihydrothiazolo[5,4-f]quinazoline-2-carbimidate (15a): white solid (72.2 mg, 88%), mp. 228–230 °C; 1H-NMR (DMSO-d6) δ 9.34 (s, 1H, NH), 8.54 (d, J = 8.7 Hz, 1H, H4), 8.53 (s, 1H, H7), 7.91 (d, J = 8.7 Hz, 1H, H5), 5.29 (sept, J = 6.3 Hz, 1H, NCH), 3.30–3.25 (m, 1H, CH), 1.42 (d, J = 6.3 Hz, 6H, CH3), 1.12–1.06 (m, 4H, CH); 13C-NMR (CDCl3) δ 163.2, 161.1, 160.7, 152.0, 147.2, 147.1, 133.2, 130.0, 126.7, 116.5, 70.4, 29.8, 21.8, 6.7 (2C) ; νmax 3275, 2971, 1668, 1641, 1586, 1494, 1455, 1352, 1313, 1145, 1109, 1054, 893, 823 cm−1; HRMS calcd for C16H17N4O2S [M + H]+ 329.1072 found 329.1065.
Isopropyl 8-(2-methoxyethyl)-9-oxo-8,9-dihydro[1,3]thiazolo[5,4-f]quinazoline-2-carboximidate (15b): white solid (56.3 mg, 65%), mp. 174–176 °C; 1H-NMR (DMSO-d6) δ 9.34 (br s, 1H, NH), 8.56 (d, J = 9.0 Hz, 1H, H4), 8.54 (s, 1H, H7), 7.92 (d, J = 9 .0 Hz, 1H, H5), 5.29 (sept, J = 7.0 Hz, 1H, OCH), 4.30 (t, J = 5.1 Hz, 2H, OCH2), 3.68 (t, J = 5.1 Hz, 2H, NCH2), 3.27 (s, 3H, OCH3), 1.41 (d, J = 7.0 Hz, 6H, 2x CH3); 13C-NMR (DMSO-d6) δ 161.5, 159.0, 158.8, 151.0, 148.9, 147.6, 131.8, 129.6, 126.8, 115.9, 69.5, 68.9, 58.1, 45.9, 21.4 (2C); νmax 3278, 2981, 2928, 1658, 1641, 1588, 1350, 1140, 1111, 1052, 889, 837 cm−1; HRMS calcd for C16H19N4O3S [M + H]+ 347.1178 found 347.1170.
Isopropyl 8-(3-methoxypropyl)-9-oxo-8,9-dihydro[1,3]thiazolo[5,4-f]quinazoline-2-carboximidate (15c): white solid (81 mg, 90%), mp. 162–164 °C; 1H-NMR (DMSO-d6) δ 9.34 (s, 1H, NH), 8.59 (s, 1H, H7), 8.55 (d, J = 9.0 Hz, 1H, H4), 7.92 (d, J = 9.0 Hz, 1H, H5), 5.28 (sept, J = 6.0 Hz, 1H, CH), 4.17 (t, J = 6.9 Hz, 2H, OCH2), 3.40 (t, J = 6.0 Hz, 2H, NCH2), 3.28 (s, 3H, OCH3), 2.00 (dt, J = 6.9, 6.0 Hz, 2H, CH2), 1.40 (d, J = 6.0 Hz, 6H, CH3), 13C-NMR (DMSO-d6) δ 161.4, 159.1, 158.9, 150.9, 148.7, 147.6, 131.7, 129.5, 126.8, 115.9, 69.5, 69.1, 57.9, 44.4, 28.2, 21.4 (2C); νmax 3275, 2993, 2939, 1663, 1582, 1340, 1112, 1058, 830 cm−1; HRMS calcd for C17H21N4O3S [M + H]+ 361.1331 found. 361.1334.
3.3. In Vitro Kinase Preparation and Assays [25]
3.3.1. Buffers
Buffer A: MgCl2 (10 mM), 1 mM ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’- tetraacetic acid (EGTA), 1 mM dithiothreitol (DTT), 25 mM Tris-HCl pH 7.5, 50 μg heparin/mL. Buffer B: β-Glycerophosphate (60 mM), 30 mM p-nitrophenylphosphate, 25 mM 3-(N-morpholino) propanesulfonic acid (Mops) (pH 7.2), 5 mM EGTA, 15 mM MgCl2, 1 mM DTT, 0.1 mM sodium vanadate.
3.3.2. Kinase Preparations and Assays
Kinase activities were assayed in triplicates in buffer A or B, for 30 min. at 30 °C, at a final adenosine triphosphate (ATP) concentration of 15 μM. Blank values were substracted and activities expressed in % of the maximal activity, i.e., in the absence of inhibitors. Controls were performed with appropriate dilutions of dimethylsulfoxide (DMSO). IC50 values were calculated from dose-response curves established by Sigma-Plot graphs. The GSK-3, CK1, DYRK1A and CLK1 peptide substrates were obtained from Proteogenix (Oberhausbergen, France).
CDK5/p25. (Human, recombinant) was prepared as previously described [21]. Its kinase activity was assayed in buffer A, with 1 mg of histone H1/mL, in the presence of 15 μM [γ-33P] ATP (3000 Ci/mmol; 10 mCi/mL) in a final volume of 30 μL. After 30 min incubation at 30 °C, 25 μL aliquots of supernatant were spotted onto sheets of P81 phosphocellulose paper (Whatman) and 20 s later, the filters were washed eight times (for at least 5 min each time) in a solution of 10 mL phosphoric acid/L of water. The wet filters were counted in the presence of 1 mL ACS (Amersham, UK) scintillation fluid.
GSK-3α/β. (Porcine brain, native) was assayed, as described for CDK5/p25 but in buffer A and using a GSK-3 specific substrate (GS-1: YRRAAVPPSPSLSRHSSPHQpSEDEEE) (pS stands for phosphorylated serine) [36].
CK1δ/ε. (Porcine brain, native) was assayed as described for CDK5/p25 but using the CK1-specific peptide substrate RRKHAAIGpSAYSITA [37].
DYRK1A. (Rat, recombinant, expressed in E. coli as a glutathione transferase (GST) fusion protein) was purified by affinity chromatography on glutathione-agarose and assayed, as described for CDK5/p25 using Woodtide (KKISGRLSPIMTEQ) (1.5μg/assay) as a substrate.
CLK1. (Human, recombinant, expressed in E. coli as GST fusion protein) was assayed in buffer A (+0.15 mg BSA/mL) with RS peptide (GRSRSRSRSRSR) (1 μg/assay).
4. Conclusions
A library of thirty-three novel thiazolo[5,4-f]quinazoline derivatives 11a–c, 12a–n, 13a–h, 14a–e, 15a–c has been rapidly prepared, using microwave-assisted technology when efficient heating was needed. In order to have an efficient route to these variously 8-substituted thiazolo[5,4-f]quinazolin-9(8H)-ones, a rational multistep synthesis of methyl 6-amino-2-cyano- benzo[d]thiazole-7-carboxylate (1) was developed and optimized to the multigram scale, affording large quantities of a versatile and efficient precursor for the various target molecules in this study. The inhibitory potency of the final products against five kinases involved in AD was evaluated. Our study demonstrates that some molecules of the 12 and 13 series described in this paper are particularly promising for the development of new multi-target inhibitors of kinases. The most active compounds showed nanomolar IC50 values for CLK1, DYRK1A and GSK-3α/β over the other tested enzymes. Although affinities of these compounds for these three kinases are not negligible, optimization is still needed and this paper allows us to consider further SAR studies for the design of more efficient inhibitors of these targeted kinases.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/5/578/s1.
Acknowledgments
Financial support from the MESR (French Ministère de l’Enseignement Supérieur & de la Recherche) is gratefully acknowledged for the doctoral fellowships to D.H.. We thank the LABEX SynOrg (ANR-11-LABX-0029) for financial support (J.G.). We also acknowledge Milestone S.r.l. (Italy) for providing the multi-mode microwave reactor (Start STM) and for technical support. This research was partly supported by grants from the ‘Fonds Unique Interministériel” (FUI) TRIAD (LM) projects, the “Fondation Jérôme Lejeune” (LM), and an FP7-KBBE-2012 grant (BlueGenics) to LM.
Author Contributions
T.B. conceived the project, helped by C.F., T.B. and D.H. designed the experiments and D.H. executed the chemical synthesis accompanied by J.G., N.L. and L.M. designed and performed the biological experiments. T.B. and C.F. wrote the paper. All authors discussed the results and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATP | Adenosine triphosphate |
| CMGC group | Group of kinases including Cyclin-dependent kinases (CDKs), Mitogen-activated protein kinases (MAP kinases), Glycogen synthase kinases (GSK) and Cyclin dependent kinases (CDK-like kinases) |
| DMF | Dimethylformamide |
| MTDL | Multi-target-directed ligand |
| NBS | N-bromosuccinimide |
| SAR | Structure Activity Relationship |
References and Notes
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Terro, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Flajolet, M.; He, G.; Heiman, M.; Lin, A.; Nairn, A.C.; Greengard, P. Regulation of Alzheimer’s disease amyloid-β formation by casein kinase I. Proc. Nat. Acad. Sci. USA 2007, 104, 4159–4164. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, H.; Metternich, R. Drug discovery process for kinase Inhibitors. ChemBioChem 2005, 6, 455–459. [Google Scholar] [CrossRef] [PubMed] this paper is the editorial of a special issue “Kinases in drug discovery”. Chem. Biol. Chem. 2005, 6, 453–574.
- Wu, P.; Nielsen, T.E.; Clausen, M.H. Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discovery Today 2016, 21, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef] [PubMed]
- For a complete review see: Harris, C.S.; Hennequin, L.; Morgentin, R.; Pasquet, G. Synthesis and functionnalization of 4-substituted quinazolines as kinases templates. In Targets in Heterocyclic Systems—Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Italian Society of Chemistry: Roma, Italy, 2010; Volume 14, pp. 315–350. [Google Scholar]
- Logé, C.; Testard, A.; Thiéry, V.; Lozach, O.; Blairvacq, M.; Robert, J.-M.; Meijer, L.; Besson, T. Novel 9-oxo-thiazolo[5,4-f]quinazoline-2-carbonitrile derivatives as dual cyclin-dependent kinase 1 (CDK1)/glycogen synthase kinase-3 (GSK-3) inhibitors: synthesis, biological evaluation and molecular modeling studies. Eur. J. Med. Chem. 2008, 43, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Testard, A.; Logé, C.; Léger, B.; Robert, J.-M.; Lozach, O.; Blairvacq, M.; Meijer, L.; Thiéry, V.; Besson, T. Thiazolo[5,4-f]quinazolin-9-ones, inhibitors of glycogen synthase kinase-3. Bioorg. Med. Chem. Lett. 2006, 16, 3419–3423. [Google Scholar] [CrossRef] [PubMed]
- Loidreau, Y.; Deau, E.; Marchand, P.; Nourrisson, M.-R.; Logé, C.; Coadou, J.M.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and molecular modelling studies of 8-arylpyrido[3’,2’:4,5]thieno[3,2-d] pyrimidin-4-amines as multitarget Ser/Thr kinases inhibitors. Eur. J. Med. Chem. 2015, 92, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-aryl-7-methoxybenzo[b]furo[3,2-d] pyrimidin-4-amines and their N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amine analogues as dual inhibitors of CLK1 and DYRK1A kinases. Eur. J. Med. Chem. 2013, 59, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Lozach, O.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors. Eur. J. Med. Chem. 2012, 58, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Foucourt, A.; Dubouilh-Benard, C.; Chosson, E.; Corbière, C.; Buquet, C.; Iannelli, M.; Leblond, B.; Marsais, F.; Besson, T. Microwave-accelerated Dimroth rearrangement for the synthesis of 4-anilino-6-nitroquinazolines. Application to an efficient synthesis of a microtubule destabilizing agent. Tetrahedron 2010, 66, 4495–4502. [Google Scholar] [CrossRef]
- Foucourt, A.; Hédou, D.; Dubouilh-Benard, C.; Désiré, L.; Casagrande, A.-S.; Leblond, B.; Loaëc, N.; Meijer, L.; Besson, T. Design and synthesis of thiazolo[5,4-f]quinazolines as DYRK1A inhibitors, Part I. Molecules 2014, 19, 15546–15571. [Google Scholar] [CrossRef] [PubMed]
- Foucourt, A.; Hédou, D.; Dubouilh-Benard, C.; Désiré, L.; Casagrande, A.-S.; Leblond, B.; Loaëc, N.; Meijer, L.; Besson, T. Design and synthesis of thiazolo[5,4-f]quinazolines as DYRK1A inhibitors, Part II. Molecules 2014, 19, 15411–15439. [Google Scholar] [CrossRef] [PubMed]
- Leblond, B.; Casagrande, A.-S.; Désiré, L.; Foucourt, A.; Besson, T. DYRK1 inhibitors and uses thereof WO 2013026806. Chem. Abstr. 2013, 158, 390018. [Google Scholar]
- Abbassi, R.; Johns, T.G.; Kassiou, M.; Munoz, L. DYRK1A in neurodegeneration and cancer: Molecular basis and clinical implications. Pharmacol. Ther. 2015, 151, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Medda, F.; Smith, B.; Gokhale, V.; Shaw, A.Y.; Dunckley, T.; Hulme, C. Beyond secretases: Kinase inhibitors for the treatment of Alzheimer's disease. Annu. Rep. Med. Chem. 2013, 48, 57–71. [Google Scholar]
- Smith, B.; Medda, F.; Gokhale, V.; Dunckley, T.; Hulme, C. Recent Advances in the Design, Synthesis, and Biological Evaluation of Selective DYRK1A Inhibitors: A New Avenue for a Disease Modifying Treatment of Alzheimer’s? ACS Chem. Neurosci. 2012, 3, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Varjosalo, M.; Keskitalo, S.; Van Drogen, A.; Nurkkala, H.; Vichalkovski, A.; Aebersold, R.; Gstaiger, M. The protein interaction landscape of the human CMGC kinase group. Cell Rep. 2013, 3, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Miralinaghi, P.; Mariano, M.; Hartmann, R.W.; Engel, M. Hydroxybenzothiophene ketones are efficient pre-mRNA splicing modulators due to dual inhibition of Dyrk1A and Clk1/4. ACS Med. Chem. Lett. 2014, 5, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Dehbi, O.; Tikad, A.; Bourg, S.; Bonnet, P.; Lozach, O.; Meijer, L.; Aadil, M.; Akssira, M.; Guillaumet, G.; Routier, S. Synthesis and optimization of an original V-shaped collection of 4–7-disubstituted pyrido[3,2-d]pyrimidines as CDK5 and DYRK1A Inhibitors. Eur. J. Med. Chem. 2014, 80, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Bajda, M.; Guzior, N.; Ignasik, M.; Malawska, B. Multi-target-directed ligands in Alzheimer’s disease treatment. Curr. Med. Chem. 2011, 18, 4949–4975. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.A.; Chitti, S.; Rajesh, B.; Prasanth, V.V.; Kishen, J.V.R.; Vali, R.K. In silico based ligand design and docking studies of GSK-3β inhibtors. Chem. Bio. Inform. J. 2010, 10, 1–10. [Google Scholar] [CrossRef]
- Alexandre, F.R.; Domon, L.; Frère, S.; Testard, A.; Thiéry, V.; Besson, T. Microwaves in drug discovery and multi-step synthesis. Mol. Divers. 2003, 7, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, F.R.; Berecibar, A.; Wrigglesworth, R.; Besson, T. Efficient synthesis of thiazoloquinazolinone derivatives. Tetrahedron Lett. 2003, 44, 4455–4458. [Google Scholar]
- Besson, T.; Guillard, J.; Rees, C.W. Multistep synthesis of thiazoloquinazolines under microwave irradiation in solution. Tetrahedron Lett. 2000, 41, 1027–1030. [Google Scholar] [CrossRef]
- Hédou, D.; Harari, M.; Godeau, J.; Dubouilh-Benard, C.; Fruit, C.; Besson, T. Synthesis of polyfunctionalized benzo[d]thiazoles as novel anthranilic acid derivatives. Tetrahedron Lett. 2015, 56, 4088–4092. [Google Scholar] [CrossRef]
- Hédou, D.; Deau, E.; Harari, M.; Sanselme, M.; Fruit, C.; Besson, T. Rational multistep synthesis of a novel polyfunctionalized benzo[d]thiazole and its thiazolo[5,4-b]pyridine analogue. Tetrahedron 2014, 70, 5541–5549. [Google Scholar] [CrossRef]
- Hédou, D.; Guillon, R.; Lecointe, C.; Logé, C.; Chosson, E.; Besson, T. Novel synthesis of angular thiazolo[5,4-f] and [4,5-h]quinazolines, preparation of their linear thiazolo[4,5-g] and [5,4-g]quinazoline analogs. Tetrahedron 2013, 69, 3182–3191. [Google Scholar] [CrossRef]
- Methyl 2-amino-5-nitrobenzoate (2) is commercially available but quite expensive. It can be efficiently synthesized from the cheaper 5-nitro- anthranilic acid [28].
- Deau, E.; Hédou, D.; Chosson, E.; Levacher, V.; Besson, T. Convenient one-pot synthesis of N3-substituted pyrido[2,3-d]-, pyrido[3,4-d]-, pyrido[4,3-d]-pyrimidin-4(3H)-ones, and quinazolin-4(3H)-ones analogs. Tetrahedron Lett. 2013, 54, 3518–3521. [Google Scholar] [CrossRef]
- Giraud, F.; Alves, G.; Debiton, E.; Nauton, L.; Thery, V.; Durieu, E.; Ferandin, Y.; Lozach, O.; Meijer, L.; Anizon, F.; Pereira, E.; Moreau, P. Synthesis, protein kinase inhibitory potencies, and in vitro antiproliferative activities of meridianin derivatives. J. Med. Chem. 2011, 54, 4474–4489. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.; Knockaert, M.; Reinhardt, J.; Lozach, O.; Schmitt, S.; Baratte, B.; Koken, M.; Coburn, S.P.; Tang, L.; Jiang., T.; Liang, D.C.; et al. Roscovitine targets, protein kinases and pyridoxal kinase. J. Biol. Chem. 2005, 280, 31208–31219. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bidd, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Mandelkow, E.-M.; Eisenbrand, G.; et al. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s Disease: A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 2001, 276, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Primot, A.; Baratte, B.; Gompel, M.; Borgne, A.; Liabeuf, S.; Romette, J.L.; Jho, E.H.; Costantini, F.; Meijer, L. Purification of GSK-3 by affinity chromatography on immobilized axin. Protein Expression Purif. 2000, 20, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, J.; Ferandin, Y.; Meijer, L. Purification of CK1 by affinity chromatography on immobilised axin. Protein Expression Purif. 2007, 54, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.K.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef]
- Jain, P.; Karthikeyan, C.; Moorthy, N.S.H.N.; Waiker, D.K.; Jain, A.K.; Trivedi, P. Human CDC2-like kinase 1 (CLK1): A novel target for Alzheimer’s disease. Curr. Drug Targets 2014, 15, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Soppa, U.; Tejedor, F.J. DYRK1A: A potential drug target for multiple Down Syndrome neuropathologies. CNS Neurol. Disord.-Drug Targets 2014, 13, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tell, V.; Hilgeroth, A. Recent developments of protein kinase inhibitors as potential AD therapeutics. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Coombs, T.C.; Tanega, C.; Shen, M.; Wang, J.L.; Auld, D.S.; Gerritz, S.W.; Schoenen, F.J.; Thomas, C.J.; Aubé, J. Small-molecule pyrimidine inhibitors of the cdc2-like (Clk) and dual specificity tyrosine phosphorylation-regulated (Dyrk) kinases: Development of chemical probe ML315. Bioorg. Med. Chem. Lett. 2013, 23, 3654–3661. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Mobashir, M.; Hoda, N. Pivotal role of glycogen kinase-3: A therapeutic target for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 107, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 12, 13, 14 and 15 are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).