Protective Effects of Dihydromyricetin against •OH-Induced Mesenchymal Stem Cells Damage and Mechanistic Chemistry
Abstract
:1. Introduction
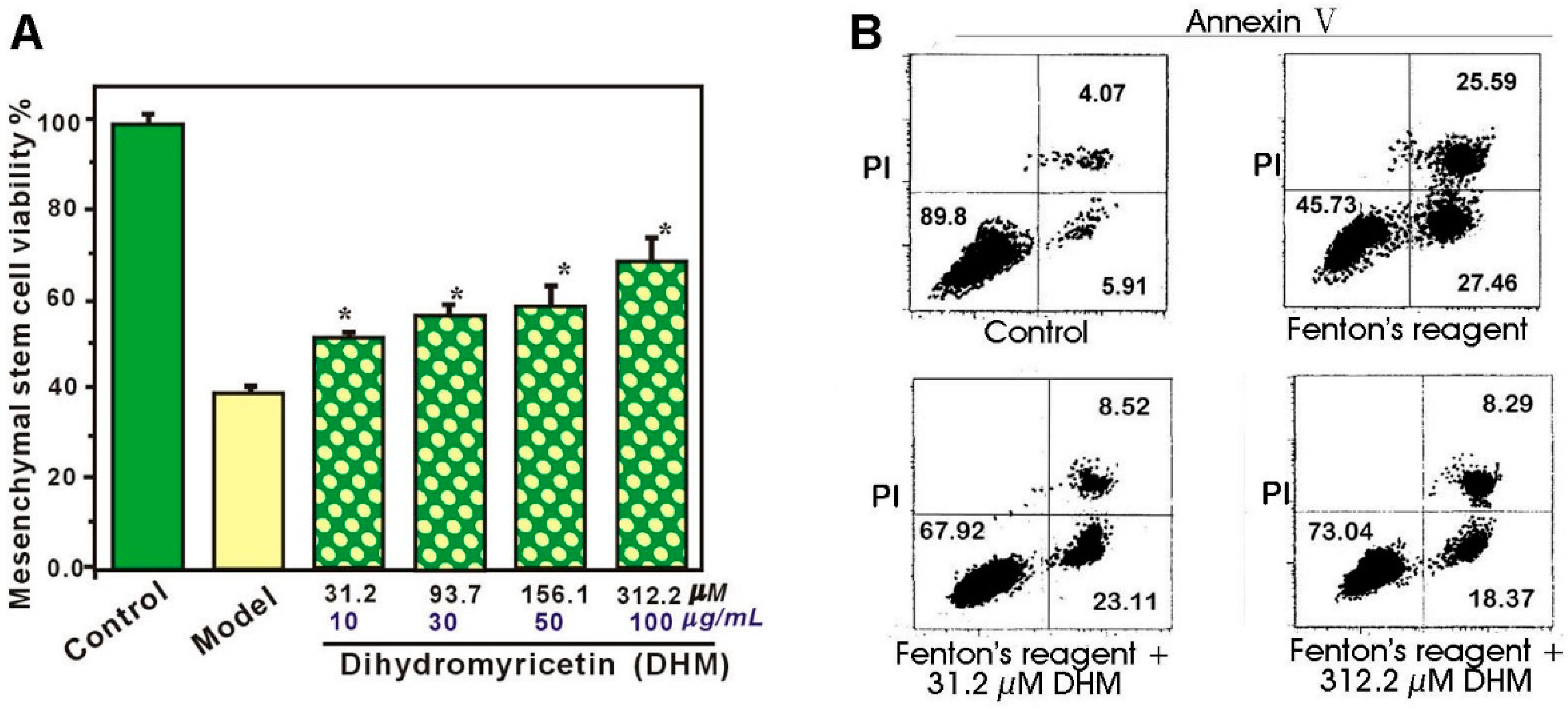
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Animals
3.2. Protective Effect Against •OH-Induced Damage to Mscs (MTT Assay)
3.3. Flow Cytometry Analysis for Annexin V and PI
3.4. Protective Effect against Hydroxyl-Induced DNA Damage (DNA Protection Assay)
3.5. Pyrogallol Autoxidation Assay for •O2− Scavenging
3.6. Cu2+-Reducing Power Assay
3.7. DPPH• Radical-Scavenging Assay
3.8. ABTS+• Radical-Scavenging Assay
3.9. Colorimetry Determination and UV Spectra Determination of Fe2+-Chelation
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid |
| BHA | butylated hydroxyanisole |
| bmMSCs | bone marrow derived mesenchymal stem cells |
| DHM | Dhydromyricetin |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl radical |
| ET | electron transfer |
| FBS | fetal bovine serum |
| Ferrozine | 3-(2-pyridyl)-5, 6-bis (4-phenylsulfonicacid)-1,2,4-triazine |
| MSCs | mesenchymal stem cells |
| MTT | [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl] |
| ROS | reactive oxygen species |
| SD | standard deviation |
| TBA | 2-thiobarbituric acid |
| TCM | Tradition Chinese Medicine |
| Tris | Tri-hydroxymethyl amino methane |
| Trolox | (±)-6-hydroxyl-2,5,7,8-tetramethlychroman-2-carboxylic acid |
References
- Kim, E.Y.; Lee, K.B.; Kim, M.K. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014, 47, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, X.; Zhang, X.; He, X.; Lai, L.; Liu, Y.; Zhu, G.; Li, W.; Li, H.; Fang, Q.; et al. The inhibitory effect of mesenchymal stem cell on blood-brain barrier disruption following intracerebral hemorrhage in rats: Contribution of TSG-6. J. Neuroinflamm. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Calió, M.L.; Marinho, D.S.; Ko, G.M.; Ribeiro, R.R.; Carbonel, A.F.; Oyama, L.M.; Ormanji, M.; Guirao, T.P.; Calió, P.L.; Reis, L.A.; et al. Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic. Biol. Med. 2014, 70, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, M.; Hesami, Z.; Jamshidzadeh, A.; Gramizadeh, B. Antioxidant Effects of Bone Marrow Mesenchymal Stem Cell against Carbon Tetrachloride-Induced Oxidative Damage in Rat Livers. Int. J. Organ. Transplant. Med. 2014, 5, 166–173. [Google Scholar] [PubMed]
- Narita, T.; Suzuki, K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail. Rev. 2015, 20, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Xiao, J.; Zheng, G.; Xing, F.; Tipoe, G.L.; Wang, X.; He, C.; Chen, Z.Y.; Liu, Y. Antioxidant treatment enhances human mesenchymal stem cell anti-stress ability and therapeutic efficacy in an acute liver failure model. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.S.; Ning, Z.X.; Yang, S.Z.; Wu, H. Antioxidation properties and mechanism of action of dihydromyricetin from Ampelopsis grossedentata. Yao Xue Xue Bao 2003, 38, 241–244. [Google Scholar] [PubMed]
- Hou, X.; Tong, Q.; Wang, W.; Xiong, W.; Shi, C.; Fang, J. Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life Sci. 2015, 130, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, H.; Duncan, S.E.; Eigel, W.N.; O’Keefe, S.F. Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 2015, 172, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T. Research Advance in Chemical Components and Pharmacological Actions of Rattan Tea. SH. J. TCM Jun. 2008, 42, 94–96. [Google Scholar]
- Chen, Y.Q.; Jiang, N.D.; Cheng, Q.; Huang, H.B.; Meng, Y.M.; Wu, C. Study on the Hypolipidemic Effect of Flavones and Dihydromyricetin From Tengcha. J. Tea Sci. 2007, 27, 221–225. [Google Scholar]
- Fang, Y.Z.; Zheng, R.L. Theory and Application of Free Radical Biology, 1st ed.; Science Press: Beijing, China, 2002; pp. 759–761. [Google Scholar]
- Zhu, H.; Luo, P.; Fu, Y. Dihydromyricetin prevents cardiotoxicity and enhances anticancer activity induced by Adriamycin. Oncotarget 2015, 6, 3254–3267. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lindemeyer, A.K.; Gonzalez, C.; Shao, X.M.; Spigelman, I. Dihydromyricetin as a novel anti-alcohol intoxication medication. J. Neurosci. 2012, 32, 390–401. [Google Scholar]
- Li, C.P.; Cao, S.W.; Yu, Y.Y. Advanced Study on Dihydromyricetin. Chem. Reag. 2010, 32, 608–612. [Google Scholar]
- Li, W.; Wu, H.; Liu, B.G.; Hou, X.D.; Wan, D.J. Highly efficient and regioselective synthesis of dihydromyricetin esters by immobilized lipase. J. Biotechnol. 2015, 199, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Zhang, Q.Y.; Li, L.Y.; Wang, B.; Zhao, Y.Y. Simultaneous determination and pharmacokinetic studies of dihydromyricetin and myricetin in rat plasma by HPLC-DAD after oral administration of Ampelopsis grossedentata decoction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 860, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Nicolay, N.H.; Lopez, P.R.; Saffrich, R.; Huber, P.E. Radio-resistant mesenchymal stem cells: Mechanisms of resistance and potential implications for the clinic. Oncotarget 2015, 6, 19366–19380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhai, W.; Zhao, M.; Li, D.; Chai, X.; Cao, X.; Meng, J.; Chen, J. Effects of Iron Overloadon the Bone Marrow Microenvironment in Mice. PLoS ONE 2015, 10, e0120219. [Google Scholar]
- Yang, S.R.; Park, J.R.; Kang, K.S. Reactive Oxygen Species in Mesenchymal Stem Cell Aging: Implication to Lung Diseases. Oxid. Med. Cell Longev. 2015, 10, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.W.; Lim, D.H.; Yi, E.S.; Choi, Y.B.; Lee, J.W.; Yoo, K.H.; Koo, H.H.; Kim, J.H.; Suh, Y.L.; Joung, Y.S.; et al. Tandem High-Dose Chemotherapy and Autologous Stem Cell Transplantation for Atypical Teratoid/Rhabdoid Tumor. Cancer Res. Treat. 2016. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Wang, H.T.; Yan, F.X.; Zheng, Y.X.; Zeng, Z.W.; Zheng, W.H. Protective effect and mechanisms of dihydromyricetin on PC12 cells induced by oxidative injury. Zhong Yao Cai 2014, 37, 1014–1020. [Google Scholar] [PubMed]
- Li, W.; Li, Q.; Guo, F.Q.; Gu, R.Q. DNA Damage Induced by X-irradiation and Reactive Oxygen Species and the Protection Against by Sinapine. Acta Phytophysiol. Sin. 1997, 23, 319–323. [Google Scholar]
- Zheng, R.L.; Shi, Y.M.; Jia, Z.J.; Zhao, C.Y.; Zhang, Q.; Tan, X.R. Fast repair of DNA radicals. Chem. Soc. Rev. 2010, 39, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Mai, W.; Chen, D.F. Chemical Study on Protective Effect Against Hydroxyl-induced DNA Damage and Antioxidant Mechanism of Myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–390. [Google Scholar] [CrossRef]
- Yang, X.M.; Wang, X.H.; Chen, L.F. Effects of dihydromyricetin on tumor necrosis factor and NF-kappaB p65 of RAU rats. Zhongguo Zhong Yao Za Zhi 2012, 37, 2612–2617. [Google Scholar] [PubMed]
- Fang, Y.Z.; Zheng, R.L. Theory and Application of Free Radical Biology, 1st ed.; Science Press: Beijing, China, 2002; pp. 98–99. [Google Scholar]
- Villata, L.S.; Berkovic, A.M.; Gonzalez, M.C.; Mártire, D.O. One-electron oxidation of antioxidants: A kinetic-thermodynamic correlation. Redox Rep. 2013, 18, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Changha, L.; Jeyong, Y. UV direct photolysis of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) in aqueous solution: Kinetics and mechanism. J. Photochem. Photobiol. A Chem. 2008, 197, 232–238. [Google Scholar]
- Aliaga, C.; Lissi, E.A. Reactions of the radical cation derived from 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) with amino acids. Kinetics and mechanism. Inter. J. Chem. Kin. 1998, 30, 565–570. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Zheng, R.L. Theory and Application of Free Radical Biology, 1st ed.; Science Press: Beijing, China, 2002; pp. 102–103. [Google Scholar]
- Sema, D.C.; Kevser, S.B.; Esma, T. Modified cupric reducing antioxidant capacity (CUPRAC) assay for measuring the antioxidant capacities of thiol-containing proteins in admixture with polyphenols. Talanta 2009, 79, 344–351. [Google Scholar]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the Activity of Phenolic Antioxidants: Theoretical Method, Analysis of Substituent Effects, and Application to Major Families of Antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Holtomo, O.; Nsangou, M.; Fifen, J.J.; Motapon, O. DFT study of the effect of solvent on the H atom transfer involved in the scavenging of the free radicals •HO2 and •O2− by caffeic acid phenethyl ester and some of its derivatives. J. Mol. Model. 2014, 20, 2509–2522. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.K.; Lopes, T.I.; Costa, R.C.; Coelho, R.G. Radical-scavenging Potential of Phenolic Compounds from Brazilian Lichens. Orbital Electron. J. Chem. 2015, 7, 99–107. [Google Scholar] [CrossRef]
- Boudiera, A.; Tournebizea, J.; Bartoszb, G.; Hanic, S.E.; Bengueddourc, R. High-performance liquid chromatographic method to evaluate the hydrogen atom transfer during reaction between 1,1-diphenyl-2-picryl-hydrazyl radical and antioxidants. Anal. Chim. Acta 2012, 711, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Hara, Y.; Steenken, S.; Simic, M.G. Antioxidant Potential of Gallocatechins. A Pulse Radiolysis and Laser Photolysis Study. J. Am. Chem. Soc. 1995, 117, 9881–9888. [Google Scholar] [CrossRef]
- Biesaga, M. Stability of bioactive polyphenols from honey during different extraction methods. Food Chem. 2013, 136, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Ma, Y.; Lin, W.; Kai, X.; Chen, M. Study on the structure–activity of dihydromyricetin and its new production. J. Therm. Anal. Calorim. 2014, 116, 241–248. [Google Scholar] [CrossRef]
- Torreggiani, A.; Tamba, M.; Trinchero, A.; Bonora, S. Copper(II)–quercetin complexes in aqueous solutions: Spectroscopic and kinetic properties. J. Mol. Struct. 2005, 744, 759–766. [Google Scholar] [CrossRef]
- Schinella, G.; Mosca, S.; Pasamar, M.A.; Muguerza, B.; Ramón, D.; Ríos, J.L. Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Res. Int. 2010, 43, 1614–1623. [Google Scholar] [CrossRef]
- Říha, M.; Karlíčková, J.; Filipský, T.; Rocha, L.; Saso, L.; Jahodář, L.; Mladěnka, P. In vitro evaluation of copper-chelating properties of flavonoids. RSC Adv. 2014, 14, 405–422. [Google Scholar] [CrossRef]
- Li, X.C.; Gao, Y.; Li, F.; Liang, A.; Xu, Z.; Bai, Y.; Mai, W.; Chen, D.F. Maclurin protects against hydroxyl radical-induced damages to mesenchymal stem cells: Antioxidant evaluation and mechanistic insight. Chem. Biol. Interact. 2014, 219, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Lin, J.; Gao, Y. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Han, W.; Mai, W. Antioxidant activity and mechanism of Tetrahydroamentoflavone in vitro. Nat. Prod. Commun. 2013, 8, 787–789. [Google Scholar]
- Lin, J.; Li, X.C.; Han, L. Folium Sennae protects against hydroxyl radical-induced DNA damage via antioxidant mechanism: An in vitro study. Bot. Stud. 2014, 55, 16–24. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.C.; Chen, L. Protective Effect Against Hydroxyl Radical-induced DNA Damage and Antioxidant Mechanism of [6]-gingerol: A Chemical Study. Bull. Korean Chem. Soc. 2014, 35, 1633–1638. [Google Scholar] [CrossRef]
- Gülçn, İ. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. 2010, 11, 210–218. [Google Scholar]
- Sample Availability: Sample of the compound dihydromyricetin is available from the authors.








| Assays | DHM | Positive Control | IC50,Trolox/IC50,DHM | IC50,Trolox/IC50, myricitrin * |
|---|---|---|---|---|
| μg/mL (μM) | Trolox, µg/mL (μM) | |||
| DNA assay | 85.7 ± 2.2 (266.7 ± 0.5 a) | 120.6 ± 0.7(483.3 ± 0.5 b) | 1.8 | 1.7 |
| ABTS+ scavenging | 4.1 ± 0.4 (12.8 ± 0.1 b) | 1.7 ± 0.1 (6.8 ± 0.1 a) | 0.5 | 1.8 |
| Cu2+-reducing | 10.7 ± 0.1(33.4 ± 2.44 a) | 10.3 ± 0.1(41.2 ± 0.9 b) | 1.3 | 3.0 |
| ·O2− scavenging | 6.7 ± 0.2 (20.0 ± 0.4 a) | 23.2 ± 2.6(90.1 ± 0.5 b) | 4.5 | 1.9 |
| DPPH· scavenging | 2.3 ± 0.8 (7.4 ± 0.3 a) | 8.78 ± 0.2 (35.1 ± 0.4 b) | 4.7 | 1.2 |
| Fe2+ chelating | 85.7 ± 1.6(266.7 ± 0.01 b) | 59.1 ± 1.3 (200.1 ± 0.5 a) ** | 0.8 | No detected |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, J.; Lin, J.; Wang, T.; Huang, J.; Lin, Y.; Chen, D. Protective Effects of Dihydromyricetin against •OH-Induced Mesenchymal Stem Cells Damage and Mechanistic Chemistry. Molecules 2016, 21, 604. https://doi.org/10.3390/molecules21050604
Li X, Liu J, Lin J, Wang T, Huang J, Lin Y, Chen D. Protective Effects of Dihydromyricetin against •OH-Induced Mesenchymal Stem Cells Damage and Mechanistic Chemistry. Molecules. 2016; 21(5):604. https://doi.org/10.3390/molecules21050604
Chicago/Turabian StyleLi, Xican, Jingjing Liu, Jian Lin, Tingting Wang, Jieyuan Huang, Yongqiang Lin, and Dongfeng Chen. 2016. "Protective Effects of Dihydromyricetin against •OH-Induced Mesenchymal Stem Cells Damage and Mechanistic Chemistry" Molecules 21, no. 5: 604. https://doi.org/10.3390/molecules21050604
APA StyleLi, X., Liu, J., Lin, J., Wang, T., Huang, J., Lin, Y., & Chen, D. (2016). Protective Effects of Dihydromyricetin against •OH-Induced Mesenchymal Stem Cells Damage and Mechanistic Chemistry. Molecules, 21(5), 604. https://doi.org/10.3390/molecules21050604







