Synthesis and Antioxidant Activity of Alkyl Nitroderivatives of Hydroxytyrosol
Abstract
:1. Introduction
2. Results
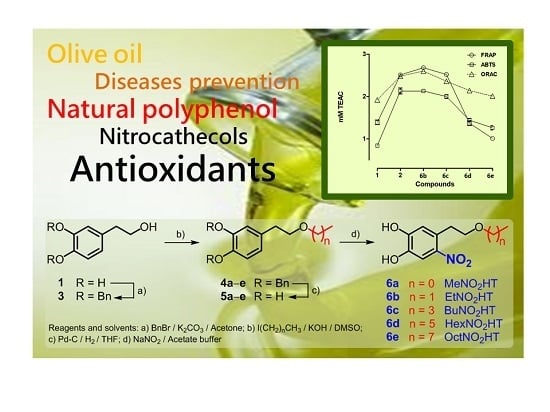
2.1. Preparation and Characterization of Alkyl Nitrohydroxytyrosyl Ethers (6a–e)
2.2. Antioxidant Activity Evaluation of Alkyl NO2HT Ethers (6b–e)
2.2.1. FRAP Assay
2.2.2. ABTS Assay
2.2.3. ORAC Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthetic Procedures
4.2.1. Synthesis of Hydroxytyrosyl Ethers (5a–e)
4.2.2. General Method of Nitration
4.3. Antioxidant Activity Determinations
4.3.1. Ferric Reducing Antioxidant Power (FRAP) Assay
4.3.2. ABTS Assay
4.3.3. Oxygen Radical Scavenging Capacity (ORAC) Assay
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| ABTS | 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| COMT | Catechol orto-Methyl Transferase |
| DMSO-d6 | hexadeuterated dimethyl sulfoxide |
| EFSA | European Food Safety Authority |
| FRAP | Ferric Reducing Antioxidant Power |
| HMBC | Hetero Multiple Bond Correlation |
| HRMS | High-Resolution Mass Spectrometry |
| HSQC | Hetero Single Quanta Correlation |
| HT | Hydroxytyrosol |
| NMR | Nuclear Magnetic Resonance |
| NO2HT | Nitrohydroxytyrosol |
| OOWW | Olive Oil Wastewaters |
| ORAC | Oxygen Radical Scavenging Capacity |
| PD | Parkinson’s Disease |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| TPTZ | 2,4,6-tri-(2-pyridyl)-1,3,5-triazine |
References
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health. Biofactors 2013, 39, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.M.; He, H.G.; Dong, M.; Zhang, Q.Y.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 24, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Kong, A.N.; Hintze, K.J.; Jeffery, E.H.; Ji, L.L.; Lei, X.G. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2011, 59, 6837–6846. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Panel on Dietetic Products, Nutrition and Allergies. EFSA J. 2011, 9, 2033–2058. [Google Scholar]
- Goya, L.; Mateos, R.; Bravo, L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells. Protection against oxidative stress induced by tert-butylhydroperoxide. Eur. J. Nutr. 2007, 46, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Ramos, S.; Granado-Serrano, A.B.; Rodríguez-Ramiro, M.; Trujillo, M.; Bravo, L.; Goya, L. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010, 54, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Gilardini, M.; Maria, S.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-Derived Compounds: A Basis for the Creation of New Pharmacological Agents for Cancer Prevention and Therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef] [PubMed]
- Madrona, A.; Pereira-Caro, G.; Mateos, R.; Rodríguez, G.; Trujillo, M.; Fernández-Bolaños, J.; Espartero, J.L. Synthesis of hydroxytyrosyl alkyl ethers from olive oil waste waters. Molecules 2009, 14, 1762–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira-Caro, G.; Madrona, A.; Bravo, L.; Espartero, J.L.; Alcudia, F.; Cert, A.; Mateos, R. Antioxidant activity evaluation of alkyl hydroxytyrosyl ethers, a new class of hydroxytyrosol derivatives. Food Chem. 2009, 115, 86–91. [Google Scholar] [CrossRef]
- Liu, L.; Jin, C.; Zhang, Y. Lipophilic phenolic compound (Lipo-PC): An emerging antioxidant applied in lipid systems. RSC Adv. 2014, 4, 2879–2891. [Google Scholar] [CrossRef]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Gordin, A.; Kaakkola, S.; Teravainen, H. Clinical advantages of COMT inhibition with entacapone—A review. J. Neural Transm. 2004, 111, 1343–1363. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, M.J.; Palma, P.; Almeida, L.; Soares-da-Silva, P. Catechol-O-methyltransferase and its inhibitors in Parkinson’s disease. CNS Drugs 2007, 13, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; de la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Gallardo, E.; Madrona, A.; Bravo, L.; Sarriá, B.; González-Correa, J.A.; Mateos, R.; Espartero, J.L. Synthesis and antioxidant activity of nitrohydroxytyrosol and its acyl derivatives. Agric. Food Chem. 2014, 62, 10297–10303. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Madrona, A.; Palma-Valdés, R.; Trujillo, M.; Espartero, J.L.; Santiago, M. The effect of hydroxytyrosol and its nitroderivatives on catechol-O-methyl transferase activity in rat striatal tissue. RSC Adv. 2014, 4, 61086–61091. [Google Scholar] [CrossRef]
- Gallardo, E.; Madrona, A.; Palma-Valdés, R.; Espartero, J.L.; Santiago, M. Effect of intracerebral hydroxytyrosol and its nitroderivatives on striatal dopamine metabolism: A study by in vivo microdialysis. Life Sci. 2015, 134, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.; Cert, A.; Espartero, J.L. New lipophilic tyrosyl esters. Comparative antioxidant evaluation with hydroxytyrosyl esters. J. Agric. Food Chem. 2008, 56, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Yaden, E.L.; Horng, J.S.; Ruffolo, R.R.; Patil, P.N.; Miller, D.D. N-Substituted imidazolines and ethylenediamines and their action on α- and β-adrenergic receptors. J. Med. Chem. 1985, 28, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Madrona, A.; Pereira-Caro, G.; Bravo, L.; Mateos, R.; Espartero, J.L. Preparation and antioxidant activity of tyrosyl and homovanillyl ethers. Food Chem. 2011, 129, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, J. Major Factors Affecting the Antioxidant of Lipids; Chan, H., Ed.; Academic Press: London, UK, 1987; pp. 141–206. [Google Scholar]
- Chimi, H.; Cillard, J.; Cillard, P.; Rahmani, M. Peroxyl radical scavenging activity of some natural phenolic antioxidants. J. Am. Oil Chem. Soc. 1991, 68, 307–312. [Google Scholar] [CrossRef]
- Porter, W.; Black, E.D.; Drolet, A.M. Use of a polyamide oxidative fluorescence test on lipid emulsions: Contrast in relative effectiveness of antioxidant in bulk versus dispersed systems. J. Agric. Food Chem. 1989, 37, 615–624. [Google Scholar] [CrossRef]
- Laguerre, M.; Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.C.; Barea, B.; Weiss, J. Chain length affects antioxidant properties of chlorogenate esters in emulsion: The cutoff theory behind the polar paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Lopez-Giraldo, L.J.; Lecomte, J.; Figueroa- Espinoza, M.C.; Barea, B.; Weiss, J.; Decker, E.A.; Villeneuve, P.J. Relationship between hydrophobicity and antioxidant ability of “phenolipids” in emulsion: A parabolic effect of the chain length of rosmarinate esters. Agric. Food Chem. 2010, 58, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Rosso, R.; Santos-Silva, M.C.; De Souza, C.A.; Licínio, M.A.; Leal, P.; Bazzo, M.L.; Yunes, M.L.; Creczynski-Pasa, T.B. Ester derivatives of gallic acid with potential toxicity toward L1210 leukemia cells. Bioorg. Med. Chem. 2008, 16, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.J.; De la Cruz, J.P.; Muñoz-Marin, J.; Guerrero, A.; Lopez-Villodres, J.A.; Madrona, A.; Espartero, J.L.; Gonzalez-Correa, J.A. Antiplatelet effect of new lipophilic hydroxytyrosol alkyl ether derivatives in human blood. Eur. J. Nutr. 2013, 52, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Marin, J.; De La Cruz, J.P.; Reyes, J.J.; López-Villodres, J.A.; Guerrero, A.; López-Leiva, I.; Espartero, J.L.; Labajos, M.T.; González-Correa, J.A. Hydroxytyrosyl alkyl ether derivatives inhibit platelet activation after oral administration to rats. Food Chem. Toxicol. 2013, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Montano, J.M.; Madrona, A.; Burgos-Moron, E.; Orta, M.L.; Mateos, R.; Espartero, J.L.; LopezLazaro, M.J. Selective cytotoxic activity of new lipophilic hydroxytyrosol alkyl ether derivatives. Agric. Food Chem. 2013, 61, 5046–5053. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Bayrasy, C.; Lecomte, L.; Chabi, B.; Decker, E.A.; Wrutniak-Cabello, C.; Cabello, G.; Villeneuve, P. How to boost antioxidants by lipophilization? Biochimie 2013, 95, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H.; Buccafusco, J.J. CNS targets for multi-functional drugs in the treatment of Alzheimer’s and Parkinson’s diseases. J. Neural Transm. 2005, 112, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bolaños, J.; Heredia, A.; Rodríguez, G.; Rodríguez, R.; Jiménez, A.; Gillen, R. Methods for Obtaining Purified Hydroxytyrosol from Products and Byproducts Derived from the Olive Tree. U.S. Patent 6849,770 B2, 1 February 2005. [Google Scholar]
- Pulido, R.; Bravo, L.; Saura-Calixto, S. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteffente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated betacyclodextrin as the solubility enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds not available.



| Position | 1 | 2 | 6a | 6b | 6c | 6d | 6e |
|---|---|---|---|---|---|---|---|
| Phenethyl Unit | |||||||
| 1 | 3.49 (t) (J1,2 = 7.2) | 3.56 (t) (J1,2 = 6.8) | 3.48 (t) (J1,2 = 6.6) | 3.51 (t) (J1,2 = 6.7) | 3.51 (t) (J1,2 = 6.7) | 3.50 (t) (J1,2 = 6.5) | 3.50 (t) (J1,2 = 6.7) |
| 2 | 2.52 (t) | 2.90 (t) | 2.99 (t) | 2.99 (t) | 2.99 (t) | 2.99 (t) | 2.99 (t) |
| 4 | 6.57 (d) (J4,8 = 2.0) | 6.75 (s) | 6.75 (s) | 6.76 (s) | 6.75 (s) | 6.75 (s) | 6.75 (s) |
| 7 | 6.60 (d) (J7,8 = 8.0) | 7.43 (s) | 7.45 (s) | 7.44 (s) | 7.44 (s) | 7.44 (s) | 7.44 (s) |
| 8 | 6.42 (dd) | ||||||
| Alkyl Chain | |||||||
| 1′ | 3.21 (s) | 3.39 (q) (3J = 7.0) | 3.33 (t) (3J = 6.5) | 3.32 (t) (3J = 6.6) | 3.22 (t) (3J = 6.6) | ||
| 2′ | 1.06 (t) | 1.42 (m) | 1.43 (m) | 1.43 (m) | |||
| 3′ | 1.26 (m) | 1.22 (m) | 1.22 (m) | ||||
| 4′ | 0.84 (t) (3J = 7.0) | ||||||
| 5′ | |||||||
| 6′ | 0.83 (t) (3J = 7.0) | ||||||
| 7′ | |||||||
| 8′ | 0.84 (t) (3J = 7.0) |
| Position | 1 | 2 | 6a | 6b | 6c | 6d | 6e |
|---|---|---|---|---|---|---|---|
| Phenethyl Unit | |||||||
| 1 | 62.5 | 61.0 | 71.7 | 69.6 | 69.8 | 69.9 | 69.9 |
| 2 | 38.4 | 36.0 | 32.5 | 32.8 | 32.8 | 32.7 | 32.7 |
| 3 | 130.1 | 127.8 | 127.3 | 127.3 | 127.4 | 127.4 | 127.4 |
| 4 | 116.2 | 118.5 | 118.4 | 118.3 | 118.4 | 118.4 | 118.3 |
| 5 | 144.8 | 150.9 | 151.0 | 151.0 | 151.0 | 151.0 | 151.0 |
| 6 | 143.2 | 143.7 | 143.9 | 143.9 | 143.8 | 143.8 | 143.8 |
| 7 | 115.3 | 112.0 | 112.0 | 112.0 | 112.0 | 112.0 | 112.0 |
| 8 | 119.3 | 139.7 | 139.6 | 139.8 | 139.8 | 139.8 | 139.7 |
| Alkyl Chain | |||||||
| 1′ | 57.8 | 65.2 | 69.6 | 69.8 | 69.8 | ||
| 2′ | 15.0 | 31.2 | 29.1 | 29.1 | |||
| 3′ | 18.8 | 25.3 | 25.6 | ||||
| 4′ | 13.7 | 31.0 | 28.7 | ||||
| 5′ | 22.0 | 28.6 | |||||
| 6′ | 13.8 | 31.2 | |||||
| 7′ | 22.0 | ||||||
| 8′ | 13.9 |
| Compound | Log Ptheor | FRAP Assay (mM) | ABTS Assay (mM) | ORAC Assay (mM) |
|---|---|---|---|---|
| 1 * | 0.96 | 1.39 ± 0.05 c | 0.84 ± 0.02 e | 1.92 ± 0.04 f |
| 2 * | 0.75 | 2.51 ± 0.04 b | 2.13 ± 0.07 a | 2.48 ± 0.04 b |
| 6b | 1.84 | 2.68 ± 0.03 a | 2.13 ± 0.03 a | 2.60 ± 0.05 a |
| 6c | 2.75 | 2.52 ± 0.04 b | 2.00 ± 0.04 b | 2.36 ± 0.04 c |
| 6d | 3.66 | 1.37 ± 0.05 c | 1.44 ± 0.04 c | 2.14 ± 0.03 d |
| 6e | 4.57 | 1.01 ± 0.03 d | 1.26 ± 0.05 d | 2.01 ± 0.03 e |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo, E.; Palma-Valdés, R.; Sarriá, B.; Gallardo, I.; De la Cruz, J.P.; Bravo, L.; Mateos, R.; Espartero, J.L. Synthesis and Antioxidant Activity of Alkyl Nitroderivatives of Hydroxytyrosol. Molecules 2016, 21, 656. https://doi.org/10.3390/molecules21050656
Gallardo E, Palma-Valdés R, Sarriá B, Gallardo I, De la Cruz JP, Bravo L, Mateos R, Espartero JL. Synthesis and Antioxidant Activity of Alkyl Nitroderivatives of Hydroxytyrosol. Molecules. 2016; 21(5):656. https://doi.org/10.3390/molecules21050656
Chicago/Turabian StyleGallardo, Elena, Rocío Palma-Valdés, Beatriz Sarriá, Irene Gallardo, José P. De la Cruz, Laura Bravo, Raquel Mateos, and José L. Espartero. 2016. "Synthesis and Antioxidant Activity of Alkyl Nitroderivatives of Hydroxytyrosol" Molecules 21, no. 5: 656. https://doi.org/10.3390/molecules21050656
APA StyleGallardo, E., Palma-Valdés, R., Sarriá, B., Gallardo, I., De la Cruz, J. P., Bravo, L., Mateos, R., & Espartero, J. L. (2016). Synthesis and Antioxidant Activity of Alkyl Nitroderivatives of Hydroxytyrosol. Molecules, 21(5), 656. https://doi.org/10.3390/molecules21050656