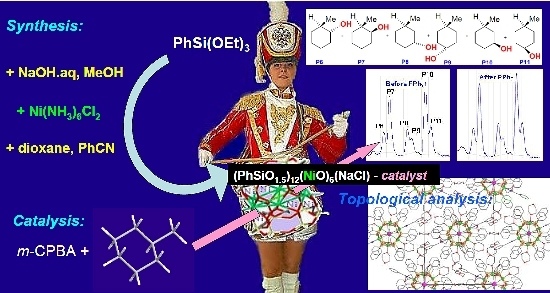
Novel Cage-Like Hexanuclear Nickel(II) Silsesquioxane. Synthesis, Structure, and Catalytic Activity in Oxidations with Peroxides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
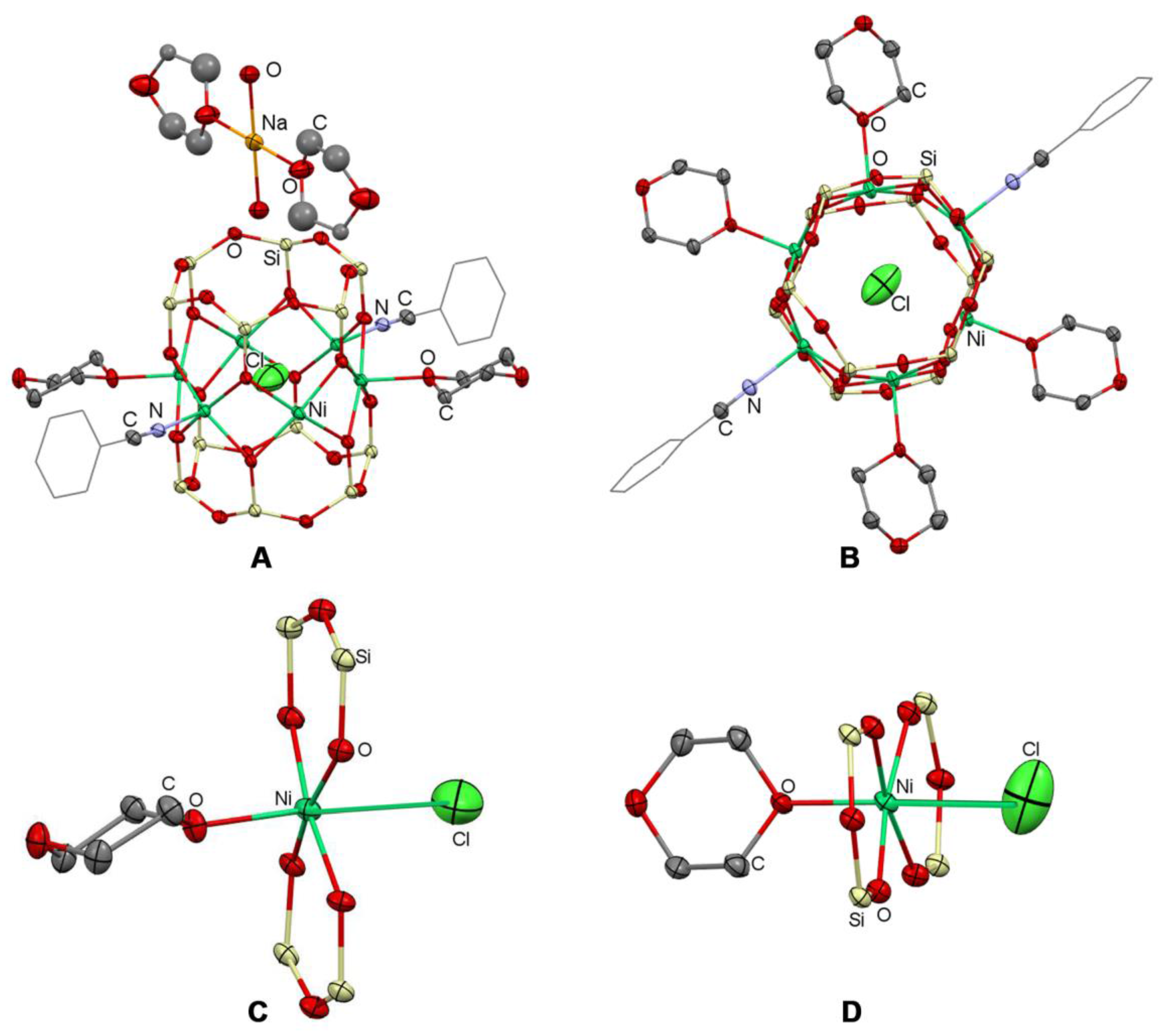
2.2. Structure
2.3. Topological Analysis and Supramolecular Assembly
2.4. Oxidations Catalyzed by Compound 1
3. Materials and Methods
3.1. Synthesis of Compound 1
3.2. X-ray Diffraction Study
3.3. Catalytic Oxidation of Alkanes and 1-Phenylethanol
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murugavel, R.; Voigt, A.; Walawalkar, M.G.; Roesky, H.W. Hetero- and Metallasiloxanes Derived from Silanediols, Disilanols, Silanetriols, and Trisilanols. Chem. Rev. 1996, 96, 2205–2236. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Fischer, A.; Gießmann, S.; Gilje, J.W.; Gun’ko, Y.; Jacob, K.; Edelmann, F.T. Disiloxanediolates and polyhedral metallasilsesquioxanes of the early transition metals and f-elements. Coord. Chem. Rev. 2000, 206–207, 321–368. [Google Scholar] [CrossRef]
- Hanssen, R.W.J.M.; van Santen, R.A.; Abbenhuis, H.C.L. The Dynamic Status Quo of Polyhedral Silsesquioxane Coordination Chemistry. Eur. J. Inorg. Chem. 2004, 675–683. [Google Scholar] [CrossRef]
- Roesky, H.W.; Anantharaman, G.; Chandrasekhar, V.; Jancik, V.; Singh, S. Control of Molecular Topology and Metal Nuclearity in Multimetallic Assemblies: Designer Metallosiloxanes Derived from Silanetriols. Chem. Eur. J. 2004, 10, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Edelmann, F.T. Metallasilsesquioxanes. Adv. Organomet. Chem. 2005, 53, 101–153. [Google Scholar]
- Jutzi, P.; Lindemann, H.M.; Nolte, J.-O.; Schneider, M. Synthesis, Structure, and Reactivity of Novel Oligomeric Titanasiloxanes. In Silicon Chemistry: From the Atom to Extended Systems; Jutzi, P., Schubert, U., Eds.; Wiley: Weinheim, Germany, 2007; pp. 372–382. [Google Scholar]
- Edelmann, F.T. Metallasilsesquioxanes. Synthetic and Structural Studies. In Silicon Chemistry: From the Atom to Extended Systems; Jutzi, P., Schubert, U., Eds.; Wiley: Weinheim, Germany, 2007; pp. 383–394. [Google Scholar]
- Ward, A.J.; Masters, A.F.; Maschmeyer, T. Metallasilsesquioxanes: Molecular Analogues of Heterogeneous Catalysts. Adv. Silicon Sci. 2011, 3, 135–166. [Google Scholar]
- Levitsky, M.M.; Bilyachenko, A.N. Modern concepts and methods in the chemistry of polyhedral metallasiloxanes. Coord. Chem. Rev. 2016, 306, 235–269. [Google Scholar] [CrossRef]
- Schax, F.; Bill, E.; Herwig, C.; Limberg, C. Dioxygen Activation by Chromium(II)- and Chromium(IV)-Siloxide Complexes. Angew. Chem. Int. Ed. 2014, 53, 12741–12745. [Google Scholar] [CrossRef] [PubMed]
- Korlyukov, A.A.; Eskova, M.A.; Tkachenko, I.M.; Kononevich, Y.N.; Shchegolikhina, O.I.; Muzafarov, A.M. Heteroligand nickel siloxane with 4-vinylbenzyl substituents. Mendeleev Commun. 2015, 25, 226–228. [Google Scholar] [CrossRef]
- Anisimov, A.A.; Kononevich, Y.N.; Zhemchugov, P.V.; Milenin, S.A.; Korlyukov, A.A.; Tsareva, U.S.; Peregudov, A.S.; Dorovatovskii, P.V.; Molodtsova, Y.A.; Takazova, R.U.; et al. Synthesis and structure of new polyhedral Ni, Na- and Cu, Na-metallasiloxanes with tolyl substituent at the silicon atom. RSC Adv. 2016, 6, 22052–22060. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Korlyukov, A.A.; Long, J.; Larionova, J.; Guari, Y.; Vologzhanina, A.V.; Eskova, M.; Shubina, E.S.; Levitsky, M.M. Unusual penta- and hexanuclear Ni(II)-based silsesquioxane polynuclear complexes. Dalton Trans. 2016, 45, 7320–7327. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, M.M.; Smirnov, V.V.; Zavin, B.G.; Bilyachenko, A.N.; Rabkina, A.Y. Metalasiloxanes: New structure formation methods and catalytic properties. Kinet. Catal. 2009, 50, 490–507. [Google Scholar] [CrossRef]
- Tan, G.; Yang, Y.; Chu, C.; Zhu, H.; Roesky, H.W. Cu24O24Si8R8: Organic Soluble 56-Membered Copper(I) Siloxane Cage and Its Use in Homogeneous Catalysis. J. Am. Chem. Soc. 2010, 132, 12231–12233. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wu, Y.; Zhu, H.; Samuel, P.P.; Roesky, H.W. Synthesis of metallasiloxanes of group 13–15 and their application in catalysis. Dalton Trans. 2013, 42, 13715–13722. [Google Scholar] [CrossRef] [PubMed]
- Beletskiy, E.V.; Shen, Z.; Riofski, M.V.; Hou, X.; Gallagher, J.R.; Miller, J.T.; Wu, Y.; Kung, H.H.; Kung, M.C. Tetrahedral Sn–silsesquioxane: Synthesis, characterization and catalysis. Chem. Commun. 2014, 50, 15699–15701. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Korlyukov, A.A.; Shul’pina, L.S.; Arkhipov, D.E.; Shubina, E.S.; Levitsky, M.M.; Kirilin, A.D.; Shul’pin, G.B. New binuclear cage-like copper(II) silsesquioxane (“cooling tower”); its high catalytic activity in oxidation of benzene and alcohols. Eur. J. Inorg. Chem. 2013, 2013, 5240–5246. [Google Scholar] [CrossRef]
- Vinogradov, M.M.; Kozlov, Y.N.; Bilyachenko, A.N.; Nesterov, D.S.; Shul’pina, L.S.; Zubavichus, Y.V.; Pombeiro, A.J.L.; Levitsky, M.M.; Yalymov, A.I.; Shul’pin, G.B. Alkane oxidation with peroxides catalyzed by cage-like copper(II) silsesquioxanes. New J. Chem. 2015, 39, 187–199. [Google Scholar] [CrossRef]
- Dronova, M.S.; Bilyachenko, A.N.; Yalymov, A.I.; Kozlov, Y.N.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; Levitsky, M.M.; Shubina, E.S.; Shul’pin, G.B. Solvent-controlled synthesis of tetranuclear cage-like copper(II) silsesquioxanes. Remarkable features of the cage structures and their high catalytic activity in oxidation with peroxides. Dalton Trans. 2014, 43, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Lamaty, F.; Bantreil, X.; Martinez, J.; Bizet, C.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; et al. Cage-like Copper(II) Silsesquioxanes: Transmetalation Reactions, Structural, Quantum Chemical and Catalytic Studies. Chem. Eur. J. 2015, 21, 8758–8770. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, G.E.; Blatov, V.A.; Proserpio, D.M. A method for topological analysis of high nuclearity coordination clusters and its application to Mn coordination compounds. Dalton Trans. 2012, 41, 4634–4640. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Wix, P.; Kostakis, G.E.; Blatov, V.A.; Proserpio, D.M.; Perlepes, S.P.; Powell, A.K. A Database of Topological Representations of Polynuclear Nickel Compounds. Eur. J. Inorg. Chem. 2013, 2013, 520–526. [Google Scholar] [CrossRef]
- Itoh, S.; Bandoh, H.; Nagatomo, S.; Kitagawa, T.; Fukuzumi, S. Aliphatic hydroxylation by a bis(μ-oxo)dinickel(III) complex. J. Am. Chem. Soc. 1999, 121, 8945–8946. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Alkane oxygenation with hydrogen peroxide catalysed by soluble derivatives of nickel and platinum. J. Chem. Res. 2002, 7, 351–353. [Google Scholar] [CrossRef]
- Nagataki, T.; Tachi, Y.; Itoh, S. NiII(TPA) as an efficient catalyst for alkane hydroxylation with m-CPBA. Chem. Commun. 2006, 4016–4018. [Google Scholar] [CrossRef] [PubMed]
- Nagataki, T.; Itoh, S. Catalytic alkane hydroxylation reaction with nickel(II) complexes supported by di- and triphenol ligands. Chem. Lett. 2007, 36, 748–749. [Google Scholar] [CrossRef]
- Nagataki, T.; Ishii, K.; Tachi, Y.; Itoh, S. Ligand effects on NiII-catalysed alkane-hydroxylation with m-CPBA. Dalton Trans. 2007, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Kunishita, A.; Doi, Y.; Kubo, M.; Ogura, T.; Sugimoto, H.; Itoh, S. Ni(II)/H2O2 reactivity in bis[(pyridin-2-yl)methyl] amine tridentate ligand system. Aromatic hydroxylation reaction by bis(μ-oxo)dinickel(III) complex. Inorg. Chem. 2009, 48, 4997–5004. [Google Scholar] [CrossRef] [PubMed]
- Wanke, R.; Guedes Da Silva, M.F.C.; Lancianesi, S.; Silva, T.F.S.; Martins, L.M.D.R.S.; Pettinari, C.; Pombeiro, A.J.L. Synthesis and coordination chemistry of a new N4-Polydentate class of pyridyl-functionalized scorpionate ligands: Complexes of FeII, ZnII, NiII, VIV, PdII and use for heterobimetallic systems. Inorg. Chem. 2010, 49, 7941–7952. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, M.; Mayilmurugan, R.; Suresh, E.; Palaniandavar, M. Nickel(II) complexes of tripodal 4N ligands as catalysts for alkane oxidation using m-CPBA as oxidant: Ligand stereoelectronic effects on catalysis. Dalton Trans. 2011, 40, 9413–9424. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, S.; Hanaue, K.; Fujimura, T.; Okuda, H.; Nakazawa, J.; Ohzu, Y.; Kobayashi, C.; Akita, M. Characterization of nickel(II)-acylperoxo species relevant to catalytic alkane hydroxylation by nickel complex with mCPBA. Dalton Trans. 2013, 42, 3346–3356. [Google Scholar] [CrossRef] [PubMed]
- Tordin, E.; List, M.; Monkowius, U.; Schindler, S.; Knör, G. Synthesis and characterisation of cobalt, nickel and copper complexes with tripodal 4N ligands as novel catalysts for the homogeneous partial oxidation of alkanes. Inorg. Chim. Acta 2013, 402, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Stephen, S.; Tembe, G. Catalytic oxyfunctionalization of alkanes and alkenes by hetero-metal Mn-Cu and Mn-Ni clusters. Indian J. Chem. 2014, 53, 1500–1504. [Google Scholar]
- Sankaralingam, M.; Balamurugan, M.; Palaniandavar, M.; Vadivelu, P.; Suresh, C.H. Nickel(II) complexes of pentadentate N5 ligands as catalysts for alkane hydroxylation by using m-CPBA as oxidant: A combined experimental and computational study. Chem. Eur. J. 2014, 20, 11346–11361. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.S.; Rocha, B.G.M.; Guedes Da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. V(IV), Fe(II), Ni(II) and Cu(II) complexes bearing 2,2,2-tris(pyrazol-1-yl)ethyl methanesulfonate: Application as catalysts for the cyclooctane oxidation. New J. Chem. 2016, 40, 528–537. [Google Scholar] [CrossRef]
- Pathan, S.; Patel, A. Solvent free clean selective oxidation of alcohols catalyzed by mono transition metal (Co, Mn, Ni)-substituted Keggin-phosphomolybdates using hydrogen peroxide. Appl. Catal. A Gen. 2013, 459, 59–64. [Google Scholar] [CrossRef]
- Adam, F.; Wan-Ting, O. Nickel porphyrin hybrid material based on functionalised silica for the selective oxidation of benzyl alcohol. J. Phys. Sci. 2013, 24, 1–19. [Google Scholar]
- Arion, V.B.; Platzer, S.; Rapta, P.; Machata, P.; Breza, M.; Vegh, D.; Dunsch, L.; Telser, J.; Shova, S.; Mac Leod, T.C.O.; et al. Marked stabilization of redox states and enhanced catalytic activity in galactose oxidase models based on transition metal S- methylisothiosemicarbazonates with -SR group in ortho position to the phenolic oxygen. Inorg. Chem. 2013, 52, 7524–7540. [Google Scholar] [CrossRef] [PubMed]
- Sarma, K.; Devi, N.; Kalita, M.; Sarma, B.; Barman, P. Nickel(II), copper(II), cobalt(II), and palladium(II) complexes with a Schiff base: Crystal structure, DFT study and copper complex catalyzed aerobic oxidation of alcohol to aldehyde. J. Coord. Chem. 2015, 68, 3685–3700. [Google Scholar] [CrossRef]
- Kosheleva, A.M.; Maksimov, N.G.; Kornienko, G.V.; Kornienko, V.L. Studies of kinetics of indirect in situ electrocatalytic oxidation of aliphatic alcohols to carboxylic acids by active forms of oxygen. Russ. J. Electrochem. 2015, 51, 1079–1085. [Google Scholar] [CrossRef]
- Urgoitia, G.; Sanmartin, R.; Herrero, M.T.; Domínguez, E. An outstanding catalyst for the oxygen-mediated oxidation of arylcarbinols, arylmethylene and arylacetylene compounds. Chem. Commun. 2015, 51, 4799–4802. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sun, L.; Yang, H.; Xu, L.; Zhang, F.; Zhu, W. Visible-light-induced aerobic photocatalytic oxidation of aromatic alcohols to aldehydes over Ni-doped NH2-MIL-125(Ti). Appl. Catal. B Environ. 2016, 187, 212–217. [Google Scholar] [CrossRef]
- Lindsay Smith, J.R.; Shul’pin, G.B. Efficient stereoselective oxygenation of alkanes by peroxyacetic acid or hydrogen peroxide and acetic acid catalysed by a manganese(IV) 1,4,7-trimethyl-1,4,7-triazacyclononane complex. Tetrahedron Lett. 1998, 39, 4909–4912. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Lindsay-Smith, J.R. Oxidations by the reagent ‘H2O2-manganese(IV) complex-carboxylic acid’. Part 1. Oxidation of saturated hydrocarbons with peroxy acids and hydrogen peroxide. Russ. Chem. Bull. 1998, 47, 2379–2386. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Stoeckli-Evans, H.; Mandelli, D.; Kozlov, Y.N.; Tesouro Vallina, A.; Woitiski, C.B.; Jimenez, R.S.; Carvalho, W.A. Oxidation of alkanes with m-chloroperbenzoic acid catalyzed by iron(III) chloride and a polydentate amine. J. Mol. Catal. A Chem. 2004, 219, 255–264. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A: Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Selectivity enhancement in functionalization of C–H bonds: A review. Org. Biomol. Chem. 2010, 8, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. C–H functionalization: Thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity. Perspective. Dalton Trans. 2013, 42, 12794–12818. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Mandelli, D.; Carvalho, W.A.; Pombeiro, A.J.L.; Shul’pin, G.B. Mild homogeneous oxidation of alkanes and alcohols including glycerol with tert-butyl hydroperoxide catalyzed by a tetracopper(II) complex. J. Catal. 2010, 272, 9–17. [Google Scholar] [CrossRef]
- Vinogradov, M.M.; Shul’pina, L.S.; Kozlov, Y.N.; Kudinov, A.R.; Ikonnikov, N.S.; Shul’pin, G.B. Oxidation of hydrocarbons and alcohols with peroxides catalyzed by new π-cymene osmium complexes. J. Organomet. Chem. 2015, 784, 52–61. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sample Availability: A sample of the compound 1 is available from the authors.






| Entry | Time (min) | Reduction with PPh3 | Cyclohexanone (M) | Cyclohexanol (M) |
|---|---|---|---|---|
| at 20 °C | ||||
| 1 | 120 | no | 0.007 | 0.007 |
| 2 | yes | 0.005 | 0.006 | |
| 3 | 300 | no | 0.009 | 0.006 |
| 4 | yes | 0.008 | 0.007 | |
| at 50 °C | ||||
| 5 | 7 | no | 0.008 | 0.02 |
| 6 | yes | 0.007 | 0.017 | |
| 7 | 15 | no | 0.009 | 0.022 |
| 8 2 | yes | 0.009 | 0.023 | |
| 9 3 | yes | 0.0002 | 0.0003 | |
| 10 4 | no | 0.0005 | 0.0027 | |
| 11 4 | yes | 0.00004 | 0.00007 | |
| 12 | 30 | no | 0.009 | 0.022 |
| 13 | yes | 0.009 | 0.022 | |
| 14 3 | yes | 0.0007 | 0.0007 | |
| 15 4 | no | 0.0005 | 0.002 | |
| 16 | 60 | no | 0.009 | 0.023 |
| 17 | yes | 0.009 | 0.023 | |
| 18 3 | yes | 0.001 | 0.001 | |
| Brutto Formula | C138H178ClN2NaNi6O52Si12 |
|---|---|
| Formula weight | 3437.54 |
| T, K | 120 |
| Space group | P21/n |
| Z | 2 |
| a, Å | 16.1899 (12) |
| b, Å | 18.2778 (14) |
| c, Å | 27.093 (2) |
| β, ° | 101.513 (2) |
| V, Å3 | 7855.9 (10) |
| dcalc, g℘cm‒3 | 1.456 |
| μ, cm‒1 | 9 |
| F(000) | 3600 |
| 2θmax, ° | 50 |
| Reflections collected | 100,379 |
| Independent reflections | 23,101 |
| Reflections with I > 2σ(I) | 11,161 |
| Parameters | 942 |
| R1 [for refl. with I > 2σ(I)] | 0.0823 |
| wR2 | 0.2050 |
| GOF | 1.006 |
| Residual electron density, e·Å‒3(ρmin/ρmax ) | 2.600/−2.575 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilyachenko, A.N.; Yalymov, A.I.; Shul’pina, L.S.; Mandelli, D.; Korlyukov, A.A.; Vologzhanina, A.V.; Es’kova, M.A.; Shubina, E.S.; Levitsky, M.M.; Shul’pin, G.B. Novel Cage-Like Hexanuclear Nickel(II) Silsesquioxane. Synthesis, Structure, and Catalytic Activity in Oxidations with Peroxides. Molecules 2016, 21, 665. https://doi.org/10.3390/molecules21050665
Bilyachenko AN, Yalymov AI, Shul’pina LS, Mandelli D, Korlyukov AA, Vologzhanina AV, Es’kova MA, Shubina ES, Levitsky MM, Shul’pin GB. Novel Cage-Like Hexanuclear Nickel(II) Silsesquioxane. Synthesis, Structure, and Catalytic Activity in Oxidations with Peroxides. Molecules. 2016; 21(5):665. https://doi.org/10.3390/molecules21050665
Chicago/Turabian StyleBilyachenko, Alexey N., Alexey I. Yalymov, Lidia S. Shul’pina, Dalmo Mandelli, Alexander A. Korlyukov, Anna V. Vologzhanina, Marina A. Es’kova, Elena S. Shubina, Mikhail M. Levitsky, and Georgiy B. Shul’pin. 2016. "Novel Cage-Like Hexanuclear Nickel(II) Silsesquioxane. Synthesis, Structure, and Catalytic Activity in Oxidations with Peroxides" Molecules 21, no. 5: 665. https://doi.org/10.3390/molecules21050665
APA StyleBilyachenko, A. N., Yalymov, A. I., Shul’pina, L. S., Mandelli, D., Korlyukov, A. A., Vologzhanina, A. V., Es’kova, M. A., Shubina, E. S., Levitsky, M. M., & Shul’pin, G. B. (2016). Novel Cage-Like Hexanuclear Nickel(II) Silsesquioxane. Synthesis, Structure, and Catalytic Activity in Oxidations with Peroxides. Molecules, 21(5), 665. https://doi.org/10.3390/molecules21050665