Carlina acaulis Exhibits Antioxidant Activity and Counteracts Aβ Toxicity in Caenorhabditis elegans
Abstract
:1. Introduction
2. Results and Discussion
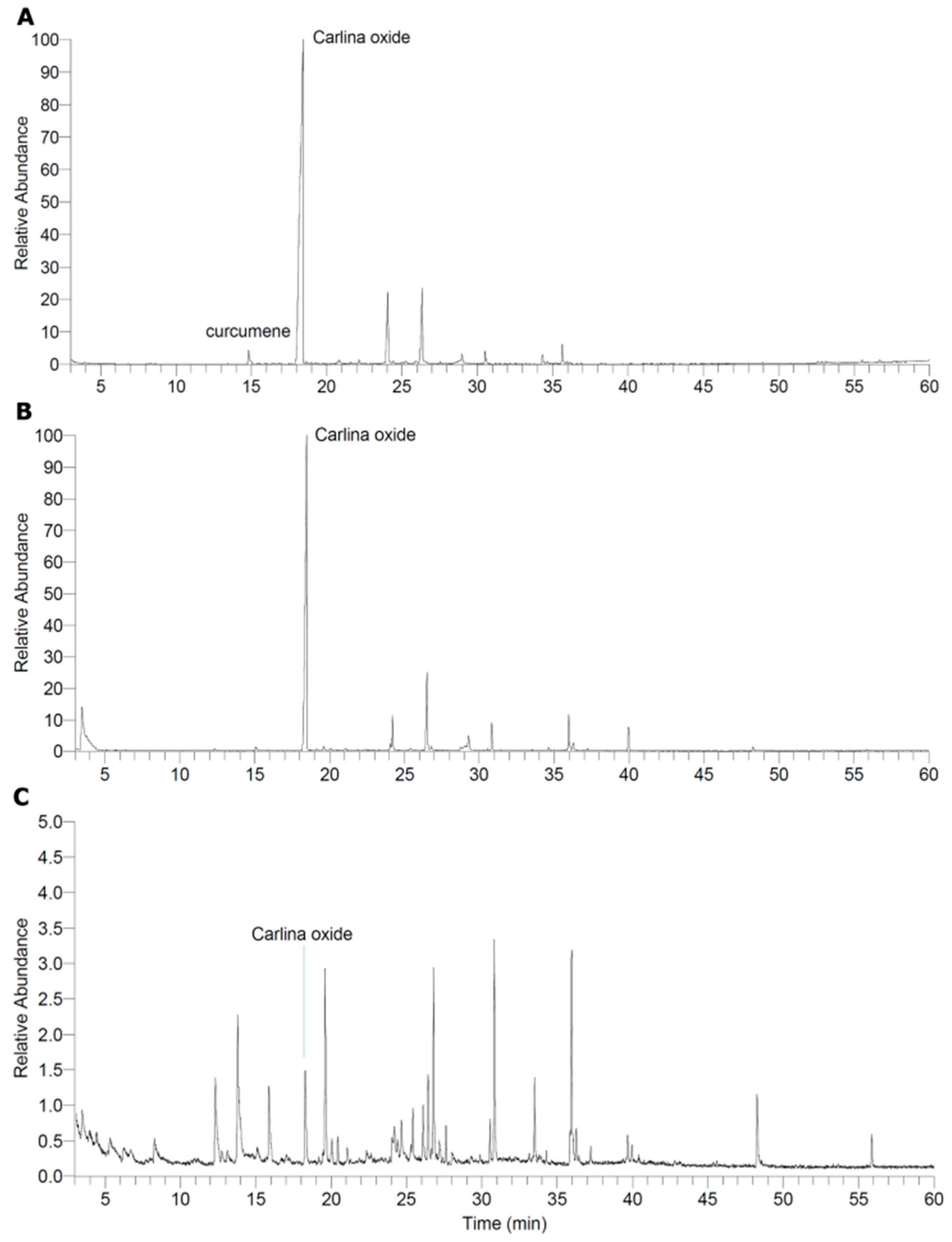
2.1. Characterisation of the Extract and Isolation of Carlina Oxide
2.2. Antioxidant Activity In Vitro
2.3. Antioxidant Activity in C. elegans
2.4. Effect Against Aβ Toxicity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. GLC-MS
3.4. DPPH●-Assay
3.5. Caenorhabditis Elegans Strains and Culture Conditions
3.6. HSP-Expression Assay
3.7. DAF-16 Delocalisation
3.8. Survival Assay
3.9. Paralysis Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| Aβ | beta-amyloid |
| CarOx | fraction of the dichloromethane extract of C. acaulis containing mostly Carlina oxide |
| DPPH● | 2,2-diphenyl-1-picrylhydracyl |
| EGCG | (−)-epigallocatechin gallate |
| EC50 | half maximal effective concentration |
| GLC-MS | gas-liquid-chromatography/mass-spectrometry |
| HSP | heat shock protein |
| PT50 | median paralysis time |
| pTLC | preparative thin layer chromatography |
References
- Meusel, H.; Kästner, A. Lebensgeschichte der Gold- und Silberdisteln: Monographie der Mediterran-Mitteleuropäischen Compositen-Gattung Carlina, Band 1; Springer-Verlag: Wien, Austria, 1990; Volume 1, p. 294. [Google Scholar]
- Schmidt-Thomé, J. Über die antibakterielle Wirkung der Silberdistelwurzel. Z. Naturforsch 1950, 5b, 409–412. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Đorđević, S.; Gorunović, M. Composition of the essential oil from the root of Carlina acaulis L. Asteraceae. J. Essent. Oil Res. 1996, 8, 577–578. [Google Scholar] [CrossRef]
- Jović, J.; Mihajilov-Krstev, T.; Žabar, A.; Stojanović-Radić, Z. Influence of solvent on antimicrobial activity of carlinae radix essential oil and decoct. Biol. Nyssana 2012, 3, 61–67. [Google Scholar]
- Herrmann, F.; Hamoud, R.; Sporer, F.; Tahrani, A.; Wink, M. Carlina oxide—A natural polyacetylene from Carlina acaulis (Asteraceae) with potent antitrypanosomal and antimicrobial properties. Planta Med. 2011, 77, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Tadić, V.; Petrović, S.; Kukić-Marković, J.; Dobrić, S.; Milenković, M.; Hadžifejzović, N. Bioactivity assays on Carlina acaulis and C. acanthifolia root and herb extracts. Dig. J. Nanomater. Biostruct. 2012, 7, 1213–1222. [Google Scholar]
- Đorđević, S.; Petrović, S.; Dobrić, S.; Milenković, M.; Vučićević, D.; Žižić, S.; Kukić, J. Antimicrobial, anti-inflammatory, anti-ulcer and antioxidant activities of Carlina acanthifolia root essential oil. J. Ethnopharmacol. 2007, 109, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Barclay, J.W.; Burgoyne, R.D.; Morgan, A. Using C. elegans to discover therapeutic compounds for ageing-associated neurodegenerative diseases. Chem. Cent. J. 2015, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Link, P.; Wetterauer, B.; Fu, Y.; Wink, M. Extracts of Glycyrrhiza uralensis and isoliquiritigenin counteract amyloid-beta toxicity in Caenorhabditis elegans. Planta Med. 2015, 81, 357–362. [Google Scholar] [PubMed]
- Rezaizadehnajafi, L.; Wink, M. Eps7630® from Pelargonium sidoides increases stress resistance in Caenorhabditis elegans probably via the daf-16/foxo pathway. Phytomedicine 2014, 21, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.Y.; Han, S.H.; Son, S.M.; Hong, H.S.; Choi, Y.J.; Byun, J.; Mook-Jung, I. Mitochondria-specific accumulation of amyloid β induces mitochondrial dysfunction leading to apoptotic cell death. PLoS ONE 2012, 7, e34929. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cuajungco, M.P.; Atwood, C.S.; Hartshorn, M.A.; Tyndall, J.D.; Hanson, G.R.; Stokes, K.C.; Leopold, M.; Multhaup, G.; Goldstein, L.E.; et al. Cu(II) potentiation of alzheimer Aβ neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999, 274, 37111–37116. [Google Scholar] [CrossRef] [PubMed]
- Opazo, C.; Huang, X.; Cherny, R.A.; Moir, R.D.; Roher, A.E.; White, A.R.; Cappai, R.; Masters, C.L.; Tanzi, R.E.; Inestrosa, N.C.; et al. Metalloenzyme-like activity of alzheimer’s disease β-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H2O2. J. Biol. Chem. 2002, 277, 40302–40308. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Wink, M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in caenorhabditis elegans. Planta Med. 2009, 75, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.T.; Johnson, T.E. Daf-16 integrates developmental and environmental inputs to mediate aging in the nematode caenorhabditis elegans. Curr. Biol. 2001, 11, 1975–1980. [Google Scholar] [CrossRef]
- McElwee, J.; Bubb, K.; Thomas, J.H. Transcriptional outputs of the Caenorhabditis elegans forkhead protein daf-16. Aging Cell 2003, 2, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kernóczi, Z.; Héthelyi, E.; Dános, B.; Tétényi, P. Presence of carlina oxide in plants of hungary. Stabilization and antimicrobial effect. Acta Pharm. Hung. 1987, 57, 171–181. [Google Scholar]
- Drake, J.; Link, C.D.; Butterfield, D.A. Oxidative stress precedes fibrillar deposition of alzheimer’s disease amyloid β-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 2003, 24, 415–420. [Google Scholar] [CrossRef]
- Cohen, E.; Bieschke, J.; Perciavalle, R.M.; Kelly, J.W.; Dillin, A. Opposing activities protect against age-onset proteotoxicity. Science 2006, 313, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Petrović, S.; Ristić, M.; Đoković, D. Composition of Carlina acanthifolia root essential oil. Chem. Nat. Compd. 2005, 41, 410–412. [Google Scholar]
- Qin, N.Y.; Yang, F.Q.; Wang, Y.T.; Li, S.P. Quantitative determination of eight components in rhizome (Jianghuang) and tuberous root (Yujin) of Curcuma longa using pressurized liquid extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Sulston, J.; Hodgkin, J. Methods. In The Nematode Caenorhabditis elegans; Wood, W.B., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1988; pp. 587–606. [Google Scholar]
- Dostal, V.; Link, C.D. Assaying β-amyloid toxicity using a transgenic C. elegans model. J. Vis. Exp. 2010, 44, 2252. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the extract or CarOx are not available.




| Treatment | PT50 ± S.E.M | Significance |
|---|---|---|
| 0.5% methanol | 36.0 ± 0.1 | |
| total extract 25 μg/mL | 36.7 ± 0.3 | |
| depleted extract 25 μg/mL | 36.3 ± 0.4 | |
| CarOx 25 μg/mL | 35.9 ± 0.6 | |
| 1% methanol | 35.0 ± 0.2 | |
| total extract 50 μg/mL | 36.6 ± 0.5 | p = 0.02 |
| depleted extract 50 μg/mL | 35.5 ± 0.4 | |
| CarOx 50 μg/mL | 36.0 ± 0.4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Link, P.; Roth, K.; Sporer, F.; Wink, M. Carlina acaulis Exhibits Antioxidant Activity and Counteracts Aβ Toxicity in Caenorhabditis elegans. Molecules 2016, 21, 871. https://doi.org/10.3390/molecules21070871
Link P, Roth K, Sporer F, Wink M. Carlina acaulis Exhibits Antioxidant Activity and Counteracts Aβ Toxicity in Caenorhabditis elegans. Molecules. 2016; 21(7):871. https://doi.org/10.3390/molecules21070871
Chicago/Turabian StyleLink, Pille, Kevin Roth, Frank Sporer, and Michael Wink. 2016. "Carlina acaulis Exhibits Antioxidant Activity and Counteracts Aβ Toxicity in Caenorhabditis elegans" Molecules 21, no. 7: 871. https://doi.org/10.3390/molecules21070871
APA StyleLink, P., Roth, K., Sporer, F., & Wink, M. (2016). Carlina acaulis Exhibits Antioxidant Activity and Counteracts Aβ Toxicity in Caenorhabditis elegans. Molecules, 21(7), 871. https://doi.org/10.3390/molecules21070871








