Synthesis of New 3-Heteroarylindoles as Potential Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
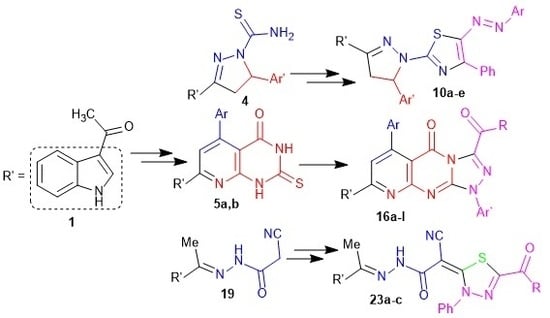
2.1. Chemistry
2.2. Biological Screening (Cytotoxic Activity)
2.3. Examination of the Compound Activities Leads to the Following Conclusions
- The activities of the synthesized compounds depend on the structural skeleton and electronic environment of the molecules.
- Based on our limited study, the 1,3,4-thiadiazole ring as in 27 has in vitro inhibitory activity greater than the 1,3-thiazole ring in 12 and more than the triazolopyridopyrimidine ring in 19.
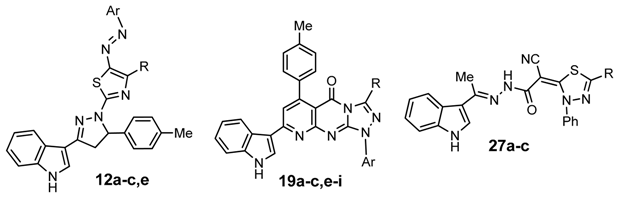
2.3.1. For the 1,3-Thiazole Ring 12a–c,e
- The in vitro inhibitory activity of the 4-methylthiazole is greater than 4-phenylthiazole (12a > 12e). This may be due to the positive inductive effect (+I effect) of the methyl group (increase activity) or the steric effect caused by phenyl group (decrease activity).
- The introduction of electron-donating group (methyl) at C4 of the phenyl group at position 4 in the 1,3-thiazole ring enhances the antitumor activity. In contrast, introduction of an electron-withdrawing group (chlorine) decreases the antitumor activity (12b > 12a > 12c).
2.3.2. For Triazolopyridopyrimidines 19a–c,e–i
- For substituent at position 3: the ester group (CO2Et) gives higher activity than the amide group (CONHPh) or the acetyl group (Ac) (19e > 19i > 19a).
- Generally, on fixing the substituents at position 3, the electron-donating group (methyl or methoxy) at C4 of the phenyl ring enhances the antitumor activity while the electron-withdrawing group (chlorine) decreases the antitumor activity (19b > 19a > 19c and 19g > 19f > 19e > 19h).
2.3.3. For 1,3,4-Thiadiazoles 27a–c
- The in vitro inhibitory activity of compounds with substituents at position 5 are in the order of: COOEt > CONHPh > CH3CO (27b > 27c > 27a).
3. Experimental
3.1. Chemistry
3.1.1. Synthesis of 3-(1H-Indol-3-yl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (5)
3.1.2. Synthesis of Thiones (7a,b)
3.1.3. Synthesis of 2-(3-(1H-Indol-3-yl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-substituted-5-(aryl diazenyl)thiazole (12a–e)
3.1.4. Alternate Synthesis of 12e
Coupling of Thiazole 15e with Benzenediazonium Chloride
3.1.5. General Procedure for the Reaction of Hydrazonoyl Halides 8 with Thiones 7a,b
3.1.6. Alternate Synthesis of 19a,e,i
3.1.7. Synthesis of N’-(1-(1H-Indol-3-yl)ethylidene)-2-cyanoacetohydrazide (22)
3.1.8. Synthesis of 3-(2-(1-(1H-Indol-3-yl)ethylidene)hydrazinyl)-2-cyano-3-oxo-N-phenylpropane thioamide (25)
3.1.9. Reaction of 25 with Hydrazonoyl Chlorides 8a,e,i
3.2. Antitumor Activity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parmentier, J.G.; Poissonnet, G.; Goldstein, S. Practical Syntheses of 5-Trifluoromethyl-1H-indoles. Heterocycles 2002, 56, 465–476. [Google Scholar]
- Black, D.S.; Craig, D.C.; Rezaie, R. Synthesis of mixed heterocalixarenes from benzofuranyl methanols and activated indoles. Chem. Commun. 2002, 8, 810–811. [Google Scholar] [CrossRef]
- Gaifa, L.; Wayne, K.N. Synthesis of novel indole analogues of mycophenolic acid as potential antineoplastic agents. Tetrahedron 2000, 56, 2583–2590. [Google Scholar]
- Aiello, F.; Valacchi, G. Synthesis and Evaluation of Indole Based Molecules for Treatment of Oxidative Stress Related Diseases. Curr. Top. Med. Chem. 2014, 14, 2576–2589. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, T.A.; Broadbent, H.S. The chemistry and pharmacology of indole-3-carbinol (indole-3-methanol) and 3-(methoxymethyl)indole. Part I. Curr. Med. Chem. 1998, 5, 337–352. [Google Scholar] [PubMed]
- Riby, J.E.; Chang, G.H.; Firestone, G.L.; Bjeldanes, L.F. Ligand-independent activation of estrogen receptor function by 3,3′-diindolylmethane in human breast cancer cells. Biochem. Pharmacol. 2000, 60, 167–177. [Google Scholar] [CrossRef]
- Hargrave, K.D.; Hess, F.K.; Oliver, J.T. N-(4-Substituted-thiazolyl)oxamic acid derivatives, new series of potent, orally active antiallergy agents. J. Med. Chem. 1983, 26, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Patt, W.C.; Hamilton, H.W.; Taylor, M.D.; Ryan, M.J.; Taylor, D.G., Jr.; Connolly, C.J.C.; Doherty, A.M.; Klutchko, S.R.; Sircar, I.; Steinbaugh, B.A.; et al. Structure-activity relationships of a series of 2-amino-4-thiazole containing renin inhibitors. J. Med. Chem. 1992, 35, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.N.; Xavier, F.P.; Vasu, K.K.; Chaturvedi, S.C.; Pancholi, S.S. Synthesis of 4-benzyl-1,3-thiazole derivatives as potential anti-inflammatory agents: An analogue-based drug design approach. J. Enzym. Inhib. Med. Chem. 2009, 24, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Jaen, J.C.; Wise, L.D.; Caprathe, B.W.; Tecle, H.; Bergmeier, S.; Humblet, C.C.; Heffner, T.G.; Meltzner, L.T.; Pugsley, T.A. 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist properties. J. Med. Chem. 1990, 33, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Ishikawa, H. Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg. Med. Chem. Lett. 1994, 4, 1601–1606. [Google Scholar] [CrossRef]
- Bell, F.W.; Cantrell, A.S.; Hoegberg, M.; Jaskunas, S.R.; Johansson, N.G.; Jordon, C.L.; Kinnick, M.D.; Lind, P.; Morin, J.M.; Noreen, R.; et al. Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J. Med. Chem. 1995, 38, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Ergenc, N.; Capan, G.; Gunay, N.S.; Ozkirimli, S.; Gungor, M.; Ozbey, S.; Kendi, E. Synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4,5-imidazolidinedione derivatives. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 343–347. [Google Scholar] [CrossRef]
- Carter, J.S.; Kramer, S.; Talley, J.J.; Penning, T.; Collins, P.; Graneto, M.J.; Seibert, K.; Koboldt, C.; Masferrer, J.; Zweifel, B. Synthesis and activity of sulfonamide-substituted 4,5-diaryl thiazoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 1171–1174. [Google Scholar] [CrossRef]
- Badorc, A.; Bordes, M.F.; de Cointet, P.; Savi, P.; Bernat, A.; Lale, A.; Petitou, M.; Maffrand, J.P.; Herbert, J.M. New orally active non-peptide fibrinogen receptor (GpIIb-IIIa) antagonists: Identification of ethyl 3-[N-[4-[4-amino[(ethoxycarbonyl) imino]methyl]phenyl]-1,3-thiazol-2-yl]-N-[1-(ethoxycarbonyl)methyl]piperid-4-yl]amino]propionate (SR 121787) as a potent and long-acting antithrombotic agent. J. Med. Chem. 1997, 40, 3393–3401. [Google Scholar] [PubMed]
- Rudolph, J.; Theis, H.; Hanke, R.; Endermann, R.; Johannsen, L.; Geschke, F.U. Seco-Cyclothialidines: New concise synthesis, inhibitory activity toward bacterial and human DNA topoisomerases, and antibacterial properties. J. Med. Chem. 2001, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Astakhov, A.V.; Chernyshev, V.M. Molecular structure of 3-amino[1,2,4]triazolo-[4,3-a] pyrimidin-5-one in various tautomeric forms: Investigation by DFT and QTAIM methods. Chem. Heterocycl. Compd. 2014, 50, 319–326. [Google Scholar] [CrossRef]
- Gomha, S.M. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4-triazolo [4,5-a]pyrimidin-5-ones. Monatshefte Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- Fares, M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Abbas, S.E.S.; Youssef, M.M.; Eladwy, R.A. Synthesis and antitumor activity of pyrido[2,3-d]pyrimidine and pyrido[2,3-d] [1,2,4]triazolo[4,3-a]pyrimidine derivatives that induce apoptosis through G(1) cell-cycle arrest. Eur. J. Med. Chem. 2014, 83, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Ahmed, S.A.; Abdelhamid, A.O. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one incorporating triazole moiety. Molecules 2015, 20, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Sun, Z.H.; Yang, M.Y.; Tan, C.X.; Weng, J.Q.; Zhang, Y.G.; Ma, Y. Microwave assistant one pot synthesis, crystal structure, antifungal activities and 3D-QSAR of novel 1,2,4-triazolo[4,3-a]pyridines. Chem. Biol. Drug Des. 2014, 84, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Badrey, M.G. Ecofriendly regioselective one-pot synthesis of chromeno[4,3-d] [1,2,4]triazolo[4,3-a]pyrimidine. Eur. J. Chem. 2013, 4, 180–184. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yang, M.Y.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 2014, 11, 1107–1111. [Google Scholar] [CrossRef]
- Gomha, S.M.; Khalil, K.D.; El-Zanate, A.M.; Riyadh, S.M. A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo[5,1-c][1,2,4]triazine, and 1,3,4-thiadiazoline. Heterocycles 2013, 87, 1109–1120. [Google Scholar]
- Yan, S.L.; Yang, M.Y.; Sun, Z.H.; Min, L.J.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole moiety. Lett. Drug Des. Discov. 2014, 11, 940–943. [Google Scholar] [CrossRef]
- Tong, J.Y.; Sun, N.B.; Wu, H.K.; Liu, X.H. Synthesis, crystal structure and biological activity of N-(5-(O-tolyl)-1,3,4-thiadiazol-2-yl)cyclopropanecarboxamide. J. Chem. Soc. Pak. 2013, 35, 1349–1353. [Google Scholar]
- Gomha, S.M.; Salah, T.A.; Abdelhamid, A.O. Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatshefte Chem. 2015, 146, 149–158. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Da, Y. Synthesis of 2-(5-(2-chlorophenyl)-2-furoylamino)-5-aryloxymethyl- 1,3,4-thiadiazoles under microwave irradiation. Synth. Commun. 2001, 31, 1829–1836. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-Aziz, H.A. Synthesis of new heterocycles derived from 3-(3-methyl-1H- indol-2-yl)-3-oxopropanenitrile as potent antifungal agents. Bull. Korean Chem. Soc. 2012, 33, 2985–2990. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Ma, Y.; Zhang, C.; Dong, W.; Pan, L.; Wang, B.; Li, Z. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropane carboxamides. Eur. J. Med. Chem. 2009, 44, 2782–2786. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Singh, A.K.; Jaiswal, N.; Singh, D. Synthesis and pharmacological activity of 1,3,4-thiadiazole derivatives: A review. Int. Res. J. Pharm. 2012, 3, 70–82. [Google Scholar]
- Gomha, S.M.; Riyadh, S.M. Synthesis under microwave irradiation of [1,2,4]triazolo[3,4-b] [1,3,4]thiadiazoles and other diazoles bearing indole moieties and their antimicrobial evaluation. Molecules 2011, 16, 8244–8256. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.L.; Li, G.; Wu, G.; Zhu, J.; Zhou, L.; Chen, P.L.; Chamberlin, A.R.; Lee, W.H. Synthesis and biological evaluation of a series of novel inhibitor of Nek2/Hec1 analogues. J. Med. Chem. 2009, 52, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Tsoua, H.; MacEwan, G.; Birnberg, G.; Grosu, G.; Bursavich, M.G.; Bard, J.; Brooijmansa, N.; Toral-Barzab, L.; Hollanderb, I.; Mansoura, T.S.; et al. Discovery and optimization of 2-(4-substituted-pyrrolo [2,3-b]pyridin-3-yl)methylene-4-hydroxybenzofuran-3(2H)-ones as potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR). Bioorg. Med. Chem. Lett. 2010, 20, 2321–2325. [Google Scholar] [CrossRef] [PubMed]
- Mavrova, A.T.; Wesselinova, D.; Tsenov, Y.A.; Denkova, P. Synthesis, cytotoxicity and effects of some 1,2,4-triazole and 1,3,4-thiadiazole derivatives on immunocompetent cells. Eur. J. Med. Chem. 2009, 44, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Oleson, J.J.; Slobada, A.; Troy, W.P.; Halliday, S.L.; Landes, M.J.; Angier, R.B.; Semb, J.; Cyr, K.; Williams, J.H. The carcinostatic activity of some 2-amino-1,3,4-thiadiazoles. J. Am. Chem. Soc. 1955, 77, 6713–6714. [Google Scholar] [CrossRef]
- Matysiak, J.; Opolski, A. Synthesis and antiproliferative activity of N-substituted 2-amino-5-(2,4- dihydroxyphenyl)-1,3,4-thiadiazoles. Bioorg. Med. Chem. 2006, 14, 4483–4489. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.F.; Khalifa, N.M.; Fahmy, H.H.; Nossier, E.S.; Abdulla, M. Design, docking, and synthesis of some new pyrazoline and pyranopyrazole derivatives as anti-inflammatory agents. J. Heterocycl. Chem. 2014, 51, 450–458. [Google Scholar] [CrossRef]
- Manna, F.; Chimenti, F.; Bolasco, A.; Bizzarri, B.; Filippelli, W.; Filippelli, A.; Gagliardi, L. Anti-inflammatory, analgesic and antipyretic 4,6-disubstituted 3-cyano-2-aminopyridines. Eur. J. Med. Chem. 1999, 34, 245–254. [Google Scholar] [CrossRef]
- Butler, R.N. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: New York, NY, USA, 1996; Volume 4, pp. 621–678. [Google Scholar]
- Huisgen, R.; Grashey, R.; Seidel, M.; Knupfer, H.; Schmidt, R. 1,3-Dipolare additionen, III. Umsetzungen des diphenylnitrilimins mit carbonyl und thiocarbonyl-verbindungen. Justus Liebigs Ann. Chem. 1962, 658, 169–180. [Google Scholar] [CrossRef]
- Mosselhi, M.A.N. A convenient synthesis of novel derivatives of pyrido[2,3-d][1,2,4]triazolo[4,3-a] pyrimidine-5,6-dione. Monatshefte Chem. 2002, 133, 1297–1304. [Google Scholar] [CrossRef]
- Eweiss, N.F.; Osman, A. Synthesis of heterocycles-2. New routes to acetylthiadiazolines and arylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Shawali, A.S.; Osman, A. Reaction of dimethylphenacylsulfonium bromide with N-nitrosoacetaryl-amides and reactions of the products with nucleophiles. Bull. Chem. Soc. Jpn. 1976, 49, 321–324. [Google Scholar] [CrossRef]
- Shawali, A.S.; Osman, A. Synthesis and reactions of phenylcarbamoyl-arylhydrazidic chlorides. Tetrahedron 1971, 27, 2517–2528. [Google Scholar] [CrossRef]
- Asiri, A.M.; Zayed, M.E.M.; Ng, S.W. Ethyl (Z)-2-chloro-2-(2-phenylhydrazin-1-ylidene)acetate. Acta Crystallogr. 2011, 67, o1962. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fu, L.; Feng, X.; Wang, J.-J.; Xun, Z.; Hu, J.-D.; Zhang, J.-J.; Zhao, Y.-W.; Huang, Z.-B.; Shi, D.-Q. Efficient Synthesis and Evaluation of Antitumor Activities of Novel Functionalized 1,8-Naphthyridine Derivatives. ACS Comb. Sci. 2015, 17, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the synthsized compounds are available from the authors.






| Compound No. | R | Ar | IC50 (µM) |
|---|---|---|---|
| 12a | CH3 | Ph | 4.92 |
| 12b | CH3 | 4-MeC6H4 | 0.95 |
| 12c | CH3 | 4-ClC6H4 | 14.52 |
| 12e | Ph | Ph | 19.44 |
| 19a | CH3CO | Ph | 6.88 |
| 19b | CH3CO | 4-MeC6H4 | 4.68 |
| 19c | CH3CO | 4-ClC6H4 | 69.85 |
| 19e | CH3CH2OCO | Ph | 4.83 |
| 19f | CH3CH2OCO | 4-MeC6H4 | 5.49 |
| 19g | CH3CH2OCO | 4-MeOC6H4 | 3.05 |
| 19h | CH3CH2OCO | 4-ClC6H4 | 18.61 |
| 19i | PhNHCO | Ph | 6.07 |
| 27a | CH3CO | Ph | 2.04 |
| 27b | CH3CH2OCO | Ph | 1.01 |
| 27c | PhNHCO | Ph | 1.27 |
| Doxorubicin | - | - | 0.75 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, A.O.; Gomha, S.M.; Abdelriheem, N.A.; Kandeel, S.M. Synthesis of New 3-Heteroarylindoles as Potential Anticancer Agents. Molecules 2016, 21, 929. https://doi.org/10.3390/molecules21070929
Abdelhamid AO, Gomha SM, Abdelriheem NA, Kandeel SM. Synthesis of New 3-Heteroarylindoles as Potential Anticancer Agents. Molecules. 2016; 21(7):929. https://doi.org/10.3390/molecules21070929
Chicago/Turabian StyleAbdelhamid, Abdou O., Sobhi M. Gomha, Nadia A. Abdelriheem, and Saher M. Kandeel. 2016. "Synthesis of New 3-Heteroarylindoles as Potential Anticancer Agents" Molecules 21, no. 7: 929. https://doi.org/10.3390/molecules21070929
APA StyleAbdelhamid, A. O., Gomha, S. M., Abdelriheem, N. A., & Kandeel, S. M. (2016). Synthesis of New 3-Heteroarylindoles as Potential Anticancer Agents. Molecules, 21(7), 929. https://doi.org/10.3390/molecules21070929