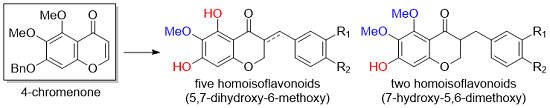
3.2. Synthesis
7-(Benzyloxy)-5,6-dimethoxychroman-4-one (16). To a solution of the acetophenone (11) (100 mg, 0.33 mmol) in toluene (2.0 mL) was added N,N-dimethylformamide dimethyl acetal (52 μL, 0.39 mmol). After stirring for 18 h at 80 °C, the mixture was cooled to 0 °C and c-HCl (0.2 mL) was added. After stirring for 1 h at 50 °C. The reaction mixture was diluted with ethyl acetate and the organic phase was washed with water and brine, and dried over anhydrous MgSO4. The solvent was removed under reduced pressure and purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford 7-(benzyloxy)-5,6-dimethoxy-4H-chromen-4-one (12) (101 mg, 97%). 1H-NMR (CDCl3, 600 MHz) δ 7.63 (d, 1H, J = 6.0 Hz), 7.46–7.40 (m, 4H), 7.37 (t, 1H, J = 6.6 Hz), 6.71 (s, 1H), 6.17 (d, 1H, J = 6.0 Hz), 5.20 (s, 2H), 3.97 (s, 3H), 3.92 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 176.2, 156.8, 154.6, 152.9, 140.7, 135.5, 128.8, 128.4, 127.2, 114.2, 113.8, 97.6, 70.9, 62.1, 61.5, 30.9. To a stirred solution of 4-chromenone (12) (10 mg, 0.03 mmol) in dry THF and Et2O (1:1) at −60 °C a solution of LiAlH4 in dry THF was added under N2 atmosphere. After stirring for 5 min, the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water and brine, and dried over anhydrous MgSO4. The solvent was removed under reduced pressure and purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the 7-(benzyloxy)-5,6-dimethoxychroman-4-one (16) (10 mg, 99%). 1H-NMR (CDCl3, 600 MHz) δ 7.43–7.37 (m, 4H), 7.35–7.33 (m, 1H), 6.29 (s, 1H), 5.13 (s, 1H), 4.42 (t, 2H, J = 6.0 Hz), 3.92 (s, 3H), 3.82 (s, 3H), 2.71 (t, 2H, J = 6.0 Hz); 13C-NMR (150 MHz, CDCl3) δ 189.1, 159.8, 158.4, 154.4, 137.7, 135.8, 128.7, 128.2, 127.2, 109.8, 97.4, 70.6, 66.8, 61.6, 61.3, 38.7.
(E)-7-(Benzyloxy)-3-(3-hydroxy-4-methoxybenzylidene)-5,6-dimethoxychroman-4-one (18a). To a solution of the 4-chromanone (16) (80 mg, 0.26 mmol) in benzene (5 mL) was added 3-hydroxy-4-methoxybenzaldehyde (58 mg, 0.38 mmol) and p-toluenesulfonic acid (7 mg, 0.03 mmol) at 0 °C. The reaction mixture was refluxed for 12 h. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the resulting 3-benzylidene-4-chromanone (18a) (60 mg, 53%). 1H-NMR (600 MHz, CDCl3) δ 7.73 (s, 1H), 7.42–7.38 (m, 4H), 7.35–7.33 (m, 1H), 6.90 (d, 1H, J = 8.4 Hz), 6.86–6.83 (m, 2H), 6.30 (s, 1H), 5.22 (d, 2H, J = 1.8 Hz), 5.13 (s, 2H), 3.99 (s, 3H), 3.94 (s, 3H), 3.85 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 179.6, 159.2, 158.2, 154.9, 147.6, 145.5, 138.1, 136.3, 135.8, 130.1, 128.7, 128.2, 128.0, 127.2, 123.2, 115.8, 110.8, 110.6, 97.5, 70.6, 67.6, 61.7, 61.3, 56.0; HRMS (EI): mass calculated for C26H24O7 [M+], 448.1522; found, 448.1521.
(E)-7-(Benzyloxy)-3-(4-hydroxy-3-methoxybenzylidene)-5,6-dimethoxychroman-4-one (18b). To a solution of the 4-chromanone (16) (80 mg, 0.26 mmol) in benzene (5 mL) was added 4-hydroxy-3-methoxybenzaldehyde (58 mg, 0.38 mmol) and p-toluenesulfonic acid (7 mg, 0.03 mmol) at 0 °C. The reaction mixture was refluxed for 12 h. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the resulting 3-benzylidene-4-chromanone (18b) (65 mg, 57%). 1H-NMR (600 MHz, CDCl3) δ 7.76 (s, 1H), 7.43–7.38 (m, 4H), 7.35–7.32 (m, 1H), 6.97 (d, 1H, J = 8.4 Hz), 6.82 (d, 1H, J = 1.8 Hz), 6.79 (dd, 1H, J = 7.8 and 1.8 Hz), 6.30 (s, 1H), 5.22 (d, 2H, J = 1.2 Hz), 5.13 (s, 2H), 3.99 (s, 3H), 3.91 (s, 3H), 3.85 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 179.5, 159.1, 158.2, 154.9, 146.9, 146.5, 138.1, 136.5, 135.8, 129.8, 128.7, 128.2, 127.2, 127.1, 123.7, 114.6, 112.7, 110.8, 97.5, 70.6, 67.6, 61.7, 61.3, 56.0; HRMS (EI): mass calculated for C26H24O7 [M+], 448.1522; found, 448.1521.
(E)-7-(Benzyloxy)-3-(3,4-bis(benzyloxy)benzylidene)-5,6-dimethoxychroman-4-one (18c). To a solution of the 4-chromanone (16) (80 mg, 0.25 mmol) in benzene (5 mL) was added 3,4-bis(benzyloxy)benzaldehyde (120 mg, 0.38 mmol) and p-toluenesulfonic acid (7 mg, 0.03 mmol) at 0 °C. The reaction mixture was refluxed for 12 h. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the resulting 3-benzylidene-4-chromanone (18c) (55 mg, 36%). 1H-NMR (600 MHz, CDCl3) δ 7.68 (s, 1H), 7.46–7.31 (m, 15H), 6.96 (d, 2H, J = 9.0 Hz), 6.82 (dd, 2H, J = 4.8 and 3.0 Hz), 6.29 (s, 1H), 5.22 (s, 2H), 5.19 (s, 2H), 5.13 (s, 2H), 5.04 (d, 2H, J = 1.8 Hz), 3.98 (s, 3H), 3.85 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 179.5, 159.1, 158.2, 154.9, 150.0, 148.4, 138.1, 136.8, 136.7, 136.1, 135.8, 130.1, 128.7, 128.6, 128.6, 128.4, 128.2, 128.0, 127.9, 127.5, 127.2, 127.2, 124.1, 116.9, 114.2, 110.8, 97.5, 71.4, 71.0, 70.6, 67.5, 61.7, 61.3; HRMS (EI): mass calculated for C39H34O7 [M+], 614.2305; found, 614.2308.
(E)-7-(Benzyloxy)-3-(4-(benzyloxy)benzylidene)-5,6-dimethoxychroman-4-one (18d). To a solution of the 4-chromanone (16) (121 mg, 0.39 mmol) in benzene (5 mL) was added 4-(benzyloxy)benzaldehyde (122 mg, 0.58 mmol) and p-toluenesulfonic acid (7 mg, 0.03 mmol) at 0 °C. The reaction mixture was refluxed for 12 h. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the resulting 3-benzylidene-4-chromanone (18d) (104 mg, 54%). 1H-NMR (600 MHz, CDCl3) δ 7.78 (s, 1H), 7.44–7.38 (m, 8H), 7.35–7.33 (m, 2H), 7.24 (d, 2H, J = 9.0 Hz), 7.24 (dd, 2H, J = 6.6 and 1.8 Hz), 6.30 (s, 1H), 5.21 (d, 2H, J = 1.8 Hz), 5.13 (s, 2H), 5.11 (s, 2H), 4.00 (s, 3H), 3.85 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 179.6, 159.6, 159.1, 158.2, 154.9, 138.1, 136.4, 136.1, 135.8, 131.7, 129.8, 128.7, 128.7, 128.2, 128.1, 127.5, 127.4, 127.2, 115.0, 110.8, 97.5, 70.6, 70.1, 67.6, 61.7, 61.3; HRMS (EI): mass calculated for C32H28O6 [M+], 508.1886; found, 508.1885.
(E)-7-(Benzyloxy)-5,6-dimethoxy-3-(4-methoxybenzylidene)chroman-4-one (18e). To a solution of the 4-chromanone (16) (82 mg, 0.26 mmol) in benzene (5 mL) was added 4-methoxybenzaldehyde (50 μL, 0.39 mmol) and p-toluenesulfonic acid (7 mg, 0.03 mmol) at 0 °C. The reaction mixture was refluxed for 12 h. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the resulting 3-benzylidene-4-chromanone (18e) (78 mg, 75%). 1H-NMR (600 MHz, CDCl3) δ 7.78 (s, 1H), 7.44–7.38 (m, 8H), 7.35–7.33 (m, 2H), 7.24 (d, 2H, J = 9.0 Hz), 7.24 (dd, 2H, J = 6.6 and 1.8 Hz), 6.30 (s, 1H), 5.21 (d, 2H, J = 1.8 Hz), 5.13 (s, 2H), 5.11 (s, 2H), 4.00 (s, 3H), 3.85 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 179.6, 159.6, 159.1, 158.2, 154.9, 138.1, 136.4, 136.1, 135.8, 131.7, 129.8, 128.7, 128.7, 128.2, 128.1, 127.5, 127.4, 127.2, 115.0, 110.8, 97.5, 70.6, 70.1, 67.6, 61.7, 61.3; HRMS (EI): mass calculated for C26H24O6 [M+], 432.1573; found, 432.1573.
7-Hydroxy-3-(3-hydroxy-4-methoxybenzyl)-5,6-dimethoxychroman-4-one (15a). A solution of the 3-benzylidene-4-chromanone (18a) (35 mg, 0.07 mmol) and 10% Pd/C (10 mg) in MeOH was placed under an atmosphere of hydrogen. After stirring for 1 h, the reaction mixture was diluted with ethyl acetate, filtered through a Celite pad, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:1) to afford the 3-benzyl-4-chromanone (15a) (22 mg, 87%). 1H-NMR (600 MHz, CD3OD) δ 6.82 (d, 1H, J = 14.4 Hz), 6.67 (d, 1H, J = 1.8 Hz), 6.63 (dd, 1H, J = 8.4 and 2.4 Hz), 6.16 (s, 1H), 4.21 (dd, 1H, J = 11.4 and 4.2 Hz), 4.04 (dd, 1H, J = 11.4 and 7.2 Hz), 3.82 (s, 3H), 3.79 (s, 3H), 3.75 (s, 3H), 3.00 (dd, 1H, J = 13.2 and 4.2 Hz), 2.66–2.60 (m, 1H), 2.58 (dd, 1H, J = 13.8 and 10.8 Hz); 13C-NMR (150 MHz, CD3OD) δ 192.4, 160.0, 158.5, 154.4, 146.3, 146.2, 136.4, 131.2, 119.9, 115.6, 111.5, 107.3, 99.1, 68.6, 60.4, 60.1, 55.0, 48.2, 32.0; HRMS (ESI): mass calcd for C19H20O7 [M + H+], 361.1281; found 361.1270.
7-Hydroxy-3-(4-hydroxy-3-methoxybenzyl)-5,6-dimethoxychroman-4-one (15b). A solution of the 3-benzylidene-4-chromanone (18b) (21 mg, 0.05 mmol) and 10% Pd/C (5 mg) in MeOH was placed under an atmosphere of hydrogen. After stirring for 1 h, the reaction mixture was diluted with ethyl acetate, filtered through a Celite pad and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:1) to afford the 3-benzyl-4-chromanone (15b) (15 mg, 73%). 1H-NMR (600 MHz, CDCl3) δ 6.84 (d, 1H, J = 8.4 Hz), 6.73–6.69 (m, 2H), 6.31 (s, 1H), 4.26 (dd, 1H, J = 11.4 and 4.2 Hz), 4.10 (dd, 1H, J = 11.4 and 7.2 Hz), 3.91 (s, 3H), 3.91 (s, 3H), 3.87 (s, 3H), 3.16 (dd, 1H, J = 13.8 and 4.2 Hz), 2.73 (m, 1H), 2.65–2.61 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ 191.6, 159.8, 155.5, 153.5, 146.5, 144.2, 135.2, 130.2, 121.9, 114.3, 111.4, 108.7, 98.8, 68.8, 61.5, 61.4, 55.9, 48.6, 32.6; HRMS (EI): mass calculated for C19H20O7 [M+], 360.1209; found, 360.1208.
3-(3,4-Dihydroxybenzyl)-7-hydroxy-5,6-dimethoxychroman-4-one (15c). A solution of the 3-benzylidene-4-chromanone (18c) (20 mg, 33 μmol) and 10% Pd/C (3.5 mg) in MeOH was placed under an atmosphere of hydrogen. After stirring for 1 h, the reaction mixture was diluted with ethyl acetate, filtered through a Celite pad and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:1) to afford the 3-benzyl-4-chromanone (15c) (11 mg, 99%). 1H-NMR (600 MHz, CDCl3) δ 6.79 (d, 1H, J = 8.4 Hz), 6.77 (d, 1H, J = 2.4 Hz), 6.63 (dd, 1H, J = 8.4 and 1.8 Hz), 6.30 (s, 1H), 4.26 (dd, 1H, J = 11.4 and 4.2 Hz), 4.09 (dd, 1H, J = 8.4 and 4.2 Hz), 3.90 (s, 3H), 3.89 (s, 3H), 3.08 (dd, 1H, J = 13.8 and 4.2 Hz), 2.70–2.67 (m, 1H), 2.62 (dd, 1H, J = 13.8 and 10.8 Hz); 13C-NMR (150 MHz, CDCl3) δ 192.2, 159.9, 155.9, 153.5, 143.8, 142.5, 135.2, 131.0, 121.6, 116.0, 115.3, 108.5, 98.9, 68.8, 61.5, 61.4, 48.5, 32.4; HRMS (EI): mass calculated for C18H18O7 [M+], 346.1053; found, 346.1056.
7-Hydroxy-3-(4-hydroxybenzyl)-5,6-dimethoxychroman-4-one (6). A solution of the 3-benzylidene-4-chromanone (18d) (23 mg, 0.05 mmol) and 10% Pd/C (5 mg) in MeOH was placed under an atmosphere of hydrogen. After stirring for 1 h, the reaction mixture was diluted with ethyl acetate, filtered through a Celite pad and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:1) to afford the 3-benzyl-4-chromanone (6) (14 mg, 94%). 1H-NMR (600 MHz, CDCl3) δ 7.09 (d, 2H, J = 8.4 Hz), 6.78 (d, 2H, J = 8.4 Hz), 6.30 (s, 1H), 4.62 (dd, 1H, J = 11.4 and 4.2 Hz), 4.08 (dd, 1H, J = 11.4 and 7.2 Hz), 3.91 (s, 3H), 3.90 (s, 3H), 3.15 (dd, 1H, J = 13.8 and 4.2 Hz), 2.73–2.69 (m, 1H), 2.66 (dd, 1H, J = 13.8 and 10.2 Hz); 13C-NMR (150 MHz, CDCl3) δ 191.7, 159.8, 155.6, 154.3, 153.5, 135.2, 130.3, 130.3, 115.4, 108.6, 98.8, 68.8, 61.5, 61.4, 48.5, 32.0; HRMS (EI): mass calculated for C18H18O6 [M+], 330.1103; found, 330.1102.
7-Hydroxy-5,6-dimethoxy-3-(4-methoxybenzyl)chroman-4-one (7). A solution of the 3-benzylidene-4-chromanone (18e) (23 mg, 0.05 mmol) and 10% Pd/C (5 mg) in MeOH was placed under an atmosphere of hydrogen. After stirring for 1 h, the reaction mixture was diluted with ethyl acetate, filtered through a Celite pad and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the 3-benzyl-4-chromanone (7) (13 mg, 80%). 1H-NMR (600 MHz, CD3COCD3) δ 7.07–7.05 (m, 2H), 6.76–6.73 (m, 2H), 6.09 (dd, 1H, J = 4.8 and 3.0 Hz), 4.14 (d, 1H, J = 4.2 Hz), 3.94 (d, 1H, J = 8.4 Hz), 3.71 (s, 3H), 3.71–3.62 (m, 6H), 3.00–3.98 (m, 1H), 2.62–2.61 (m, 1H), 2.53–2.49 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ 190.0, 159.6, 158.5, 157.1, 154.7, 136.3, 160.8, 130.8, 130.1, 113.9, 108.5, 99.1, 69.1, 60.8, 60.6, 54.6, 48.3, 31.5; HRMS (EI): mass calculated for C19H20O6 [M+], 344.1260; found, 344.1257.
7-Hydroxy-3-(3-hydroxy-4-methoxybenzyl)-5,6-dimethoxychroman-4-one (2). To a solution of the 7-hydroxy-5,6-dimethoxy-4-chromanone (15b) (10 mg, 0.03 mmol) in CH2Cl2 (5 mL) was added TMSI (5 μL, 0.09 mmol) at 0 °C for 1 h. The mixture was concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the 5,7-dihydroxy-6-methoxy-4-chromanone (2) (7.1 mg, 71%). 1H-NMR (600 MHz, CD3OD) δ 6.81 (d, 1H, J = 1.8 Hz), 6.73 (d, 1H, J = 7.8 Hz), 6.67 (dd, 1H, J = 8.4 and 1.8 Hz), 5.90 (s, 1H), 4.25 (dd, 1H, J = 11.4 and 4.2 Hz), 4.09 (dd, 1H, J = 11.4 and 7.2 Hz), 3.83 (s, 3H), 3.77 (s, 3H), 3.12 (dd, 1H, J = 13.8 and 4.8 Hz), 2.86–2.81 (m, 1H), 2.68 (d, 1H, J = 10.2 Hz); 13C-NMR (150 MHz, CD3OD) δ 199.3, 160.1, 159.3, 156.0, 148.2, 145.5, 130.0, 129.6, 121.9, 115.4, 112.8, 102.1, 95.0, 69.5, 60.1, 55.5, 47.3, 32.7; HRMS (EI): mass calculated for C18H18O7 [M+], 346.1053; found, 346.1054.
3-(3,4-Dihydroxybenzyl)-5,7-dihydroxy-6-methoxychroman-4-one (3). To a solution of the 7-hydroxy-5,6-dimethoxy-4-chromanone (15c) (20 mg, 0.03 mmol) in CH2Cl2 (5 mL) was added TMSI (5 μL, 0.09 mmol) at 0 °C for 1 h. The mixture was concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the 5,7-dihydroxy-6-methoxy-4-chromanone (3) (12 mg, 99%). 1H-NMR (600 MHz, CD3OD) δ 6.71 (d, 1H, J = 8.4 Hz), 6.67 (d, 1H, J = 2.4 Hz), 6.55 (dd, 1H, J = 7.8 and 1.8 Hz), 5.91 (s, 1H), 4.24 (dd, 1H, J = 11.4 and 4.2 Hz), 4.08 (dd, 1H, J = 11.4 and 7.2 Hz), 3.77 (s, 3H), 3.05 (dd, 1H, J = 13.8 and 4.2 Hz), 2.80–2.75 (m, 1H), 2.60 (dd, 1H, J = 13.8 and 10.2 Hz); 13C-NMR (150 MHz, CD3OD) δ 200.0, 160.5, 159.9, 156.7, 146.2, 144.9, 130.6, 130.2, 121.2, 116.9, 116.2, 102.8, 95.5, 70.0, 60.7, 47.9, 33.0; HRMS (EI): mass calculated for C17H16O7 [M+], 332.0896; found, 332.0898.
5,7-Dihydroxy-3-(4-hydroxybenzyl)-6-methoxychroman-4-one (4). To a solution of the 7-hydroxy-5,6-dimethoxy-4-chromanone (6) (10 mg, 0.03 mmol) in CH2Cl2 (5 mL) was added TMSI (5 μL, 0.09 mmol) at 0 °C for 1 h. The mixture was concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the resulting 5,7-dihydroxy-6-methoxy-4-chromanone (4) (9.8 mg, 86%). 1H-NMR (600 MHz, CD3OD) δ 7.06 (d, 2H, J = 8.4 Hz), 6.73 (d, 2H, J = 8.4 Hz), 5.90 (s, 1H), 4.23 (dd, 1H, J = 11.4 and 4.2 Hz), 4.07 (dd, 1H, J = 10.8 and 7.2 Hz), 3.77 (s, 3H), 3.10 (dd, 1H, J = 13.8 and 4.8 Hz), 2.82–2.77 (m, 1H), 2.67 (dd, 1H, J = 13.8 and 10.2 Hz); 13C-NMR (150 MHz, CD3OD) δ 199.8, 160.4, 159.7, 156.8, 156.5, 130.7, 130.0, 129.7, 116.0, 102.5, 95.4, 69.8, 60.5, 47.8, 32.6; HRMS (EI): mass calculated for C17H16O6 [M+], 316.0947; found, 316.0945.
5,7-Dihydroxy-6-methoxy-3-(4-methoxybenzyl)chroman-4-one (5). To a solution of the 7-hydroxy-5,6-dimethoxy-4-chromanone (7) (19 mg, 0.06 mmol) in CH2Cl2 (5 mL) was added TMSI (10 μL, 0.18 mmol) at 0 °C for 1 h. The mixture was concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:2) to afford the resulting 5,7-dihydroxy-6-methoxy-4-chromanone (5) (15 mg, 80%). 1H-NMR (600 MHz, CD3OD) δ 7.15 (d, 2H, J = 8.4 Hz), 6.86 (d, 2H, J = 8.4 Hz), 5.90 (s, 1H), 4.23 (dd, 1H, J = 11.4 and 4.2 Hz), 4.06 (dd, 1H, J = 11.4 and 7.8 Hz), 3.77 (s, 3H), 3.76 (s, 3H), 3.13 (dd, 1H, J = 13.8 and 4.8 Hz), 2.84–2.79 (m, 1H), 2.70 (dd, 1H, J = 13.8 and 10.2 Hz); 13C-NMR (150 MHz, CD3OD) δ 200.1, 160.7, 160.1, 160.0, 156.9, 131.4, 131.2, 130.4, 115.1, 103.0, 95.8, 70.3, 61.0, 55.7, 48.1, 32.9; HRMS (EI): mass calculated for C18H18O6 [M+], 330.1103; found, 330.1102.
(E)-7-Hydroxy-5,6-dimethoxy-3-(4-methoxybenzylidene)chroman-4-one (19). A solution of the 3-(4-methoxybenzylidene)-4-chromanone (18e) (37 mg, 0.09 mmol) and 3% Pd/C (4.8 mg) in MeOH was placed under an atmosphere of hydrogen. After stirring for 5 min, the reaction mixture was diluted with ethyl acetate, filtered through a Celite pad and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:3) to afford the debenzylated 3-(4-methoxybenzylidene)-4-chromanone (19) (11 mg, 35%). 1H-NMR (600 MHz, CDCl3) δ 7.78 (s, 1H), 7.26 (d, 2H, J = 7.8 Hz), 6.96 (d, 2H, J = 9.0 Hz), 6.31 (s, 1H), 5.22 (d, 2H, J = 1.8 Hz), 3.97 (s, 3H), 3.94 (s, 3H), 3.85 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 179.7, 160.4, 159.4, 155.3, 153.8, 136.2, 135.4, 131.7, 129.6, 127.1, 114.1, 110.4, 98.9, 67.4, 61.5, 61.4, 55.3; HRMS (EI): mass calculated for C19H18O6 [M+], 342.1103; found, 342.1106.
(E)-5,7-Dihydroxy-6-methoxy-3-(4-methoxybenzylidene)chroman-4-one (8). To a solution of the 3-(4-methoxybenzylidene)-4-chromanone (19) (15 mg, 0.04 mmol) in CH2Cl2 (5 mL) was added TMSI (13 μL, 0.09 mmol) at 0 °C for 1 h. The mixture was concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (ethyl acetate:n-hexane = 1:3) to afford the resulting 5,7-dihydroxy-6-methoxy-4-chromanone (8) (9.1 mg, 63%). 1H-NMR (600 MHz, CDCl3) δ 7.80 (s, 1H), 7.27 (d, 2H, J = 7.2 Hz), 6.97 (d, 2H, J = 8.4 Hz), 6.03 (s, 1H), 5.28 (d, 2H, J = 1.8 Hz), 3.95 (s, 3H), 3.86 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 185.9, 160.8, 157.8, 157.2, 155.2, 137.2, 132.0, 128.4, 127.3, 126.7, 114.2, 103.3, 94.0, 67.4, 60.9, 55.4; HRMS (EI): mass calculated for C18H16O6 [M+], 328.0947; found, 328.0945.