Porphyromonas gingivalis Lipopolysaccharide Induced Proliferation and Activation of Natural Killer Cells in Vivo
Abstract
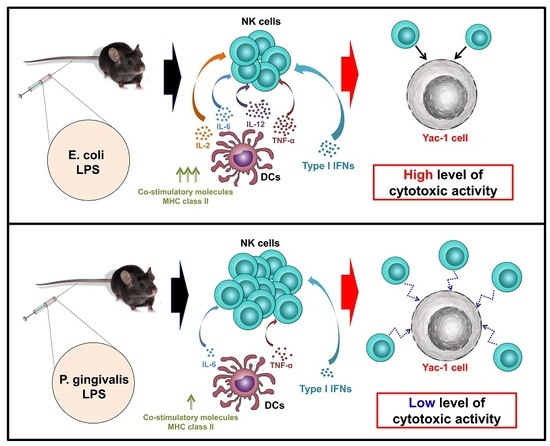
:1. Introduction
2. Results
2.1. P. Gingivalis LPS Promotes Proliferation of Spleen and sLN NK Cells but Not Blood NK Cells in Vivo
2.2. P. Gingivalis LPS Did Not Upregulate IFN-γ Production and CD69 Expression in NK Cells
2.3. P. Gingivalis LPS Induced Minimal Activation of Spleen DCs
2.4. P. Gingivalis LPS Failed to Induce IL-2, IL-12 and IL-18 Production in Spleen DCs
2.5. P. Gingivalis LPS Induced Minimal Levels of Cytotoxic Activity in Spleen NK Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Antibodies and Reagents
4.3. NK Cell Analysis
4.4. DC Analysis
4.5. Cytotoxicity Assay
4.6. Ex Vivo Cell Stimulation and Intracellular Cytokine Staining
4.7. Real-Time qPCR
4.8. ELISA Assay
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hussain, M.; Stover, C.M.; Dupont, A. P. gingivalis in Periodontal Disease and Atherosclerosis - Scenes of Action for Antimicrobial Peptides and Complement. Front. Immunol. 2015, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Berezow, A.B.; Ernst, R.K.; Coats, S.R.; Braham, P.H.; Karimi-Naser, L.M.; Darveau, R.P. The structurally similar, penta-acylated lipopolysaccharides of Porphyromonas gingivalis and Bacteroides elicit strikingly different innate immune responses. Microb. Pathog. 2009, 47, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Shimoyama, A.; Saeki, A.; Kitayama, N.; Kasamatsu, C.; Tsutsui, H.; Fukase, K. Innate immunomodulation by lipophilic termini of lipopolysaccharide; synthesis of lipid As from Porphyromonas gingivalis and other bacteria and their immunomodulative responses. Mol. BioSyst. 2013, 9, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Jitprasertwong, P.; Charadram, N.; Kumphune, S.; Pongcharoen, S.; Sirisinha, S. Female sex hormones modulate Porphyromonas gingivalis lipopolysaccharide-induced Toll-like receptor signaling in primary human monocytes. J. Periodontal Res. 2015, 51, 395–406. [Google Scholar] [CrossRef] [PubMed]
- McGuire, V.A.; Arthur, J.S. Subverting Toll-Like Receptor Signaling by Bacterial Pathogens. Front. Immunol. 2015, 6, 607. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Ertlschweiger, S.; Moritz, A.; Bantleon, H.P.; Rausch-Fan, X. Different effects of P. gingivalis LPS and E. coli LPS on the expression of interleukin-6 in human gingival fibroblasts. Acta Odontol. Scand. 2014, 72, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Desta, T.; Raptis, M.; Darveau, R.P.; Graves, D.T. P. gingivalis and E. coli lipopolysaccharides exhibit different systemic but similar local induction of inflammatory markers. J. Periodontol. 2008, 79, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.A.; Attard, T.J.; Laughton, K.M.; Mansell, A.; O’Brien-Simpson, N.M.; Reynolds, E.C. Porphyromonas gingivalis lipopolysaccharide weakly activates M1 and M2 polarized mouse macrophages but induces inflammatory cytokines. Infect. Immun. 2014, 82, 4190–4203. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yan, X.; Dong, Z.; Chen, W.; Lin, Z.T.; Hu, Q.G. Differential roles of Porphyromonas gingivalis lipopolysaccharide and Escherichia coli lipopolysaccharide in maturation and antigen-presenting functions of dentritic cells. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2482–2492. [Google Scholar] [PubMed]
- Pulendran, B.; Kumar, P.; Cutler, C.W.; Mohamadzadeh, M.; van Dyke, T.; Banchereau, J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 2001, 167, 5067–5076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, L.; Li, S.; Gu, Z.; Yan, J. Lipopolysaccharide (LPS) of Porphyromonas gingivalis induces IL-1beta, TNF-alpha and IL-6 production by THP-1 cells in a way different from that of Escherichia coli LPS. Innate Immun. 2008, 14, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Martin, M.; Schifferle, R.E.; Genco, R.J. Counteracting interactions between lipopolysaccharide molecules with differential activation of toll-like receptors. Infect. Immun. 2002, 70, 6658–6664. [Google Scholar] [CrossRef] [PubMed]
- Degli-Esposti, M.A.; Smyth, M.J. Close encounters of different kinds: Dendritic cells and NK cells take centre stage. Nat. Rev. Immunol. 2005, 5, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, K.; Riley, E.M. Innate immune response to malaria: Rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 2002, 169, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fonseca-Guimaraes, F.; Adib-Conquy, M.; Cavaillon, J.M. Natural killer (NK) cells in antibacterial innate immunity: Angels or devils? Mol. Med. 2012, 18, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Gerosa, F.; Baldani-Guerra, B.; Nisii, C.; Marchesini, V.; Carra, G.; Trinchieri, G. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 2002, 195, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Takahashi, H.; Okabe, H.; Ozaki, Y.; Hara, Y.; Kato, I. Distribution of natural killer cells in periodontal diseases: An immunohistochemical study. J. Periodontol. 1992, 63, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Stelin, S.; Ramakrishan, H.; Talwar, A.; Arun, K.V.; Kumar, T.S. Immunohistological analysis of CD1a langerhans cells and CD57 natural killer cells in healthy and diseased human gingival tissue: A comparative study. J. Indian Soc. Periodontol. 2009, 13, 150–154. [Google Scholar] [PubMed]
- Kramer, B.; Kebschull, M.; Nowak, M.; Demmer, R.T.; Haupt, M.; Korner, C.; Perner, S.; Jepsen, S.; Nattermann, J.; Papapanou, P.N. Role of the NK cell-activating receptor CRACC in periodontitis. Infect. Immun. 2013, 81, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Uchida, T.; Efron, P.A.; Scumpia, P.O.; Verma, A.; Matsumoto, T.; Tschoeke, S.K.; Ungaro, R.F.; Ono, S.; Seki, S.; et al. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J. Leukoc. Biol. 2005, 78, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Fuchs, A.; Colonna, M.; Caligiuri, M.A. NK cell and DC interactions. Trends Immunol. 2004, 25, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Zhang, W.; Du, J.Y.; Wong, K.W.; Oda, T.; Yu, Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE 2014, 9, e99396. [Google Scholar] [CrossRef] [PubMed]
- Granucci, F.; Zanoni, I.; Pavelka, N.; van Dommelen, S.L.; Andoniou, C.E.; Belardelli, F.; Degli Esposti, M.A.; Ricciardi-Castagnoli, P. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J. Exp. Med. 2004, 200, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, D.; Majewski, P.; Schulte, L.C.; Korn, J.M.; Young, R.A.; Lander, E.S.; Hacohen, N. The plasticity of dendritic cell responses to pathogens and their components. Science 2001, 294, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, G.; Pack, M.; Thomas, D.; Paludan, C.; Schmid, D.; Strowig, T.; Bougras, G.; Muller, W.A.; Moretta, L.; Munz, C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. USA 2004, 101, 16606–16611. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Willis, D.L.; Liu, M.; Purkall, D.B.; Sukumar, S.; Barbour, S.E.; Schenkein, H.A.; Tew, J.G. Dendritic-NK cell interactions in P. gingivalis-specific responses. J. Dent. Res. 2005, 84, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Shibazaki, M.; Nakamura, M.; Takada, H. Contrasting effects of lipopolysaccharides (endotoxins) from oral black-pigmented bacteria and Enterobacteriaceae on platelets, a major source of serotonin, and on histamine-forming enzyme in mice. J. Infect. Dis. 1997, 175, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Kang, J.H.; Kim, H.J.; Kim, H.J.; Kim, H.H.; Kim, J.Y.; Lee, Y. Bortezomib Inhibits Osteoclastogenesis and Porphyromonas gingivalis Lipopolysaccharide-induced Alveolar Bone Resorption. J. Dent. Res. 2015, 94, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Boeker, M.; Kapp, A. Rapid expression of the CD69 antigen on T cells and natural killer cells upon antigenic stimulation of peripheral blood mononuclear cell suspensions. Allergy 1997, 52, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Fric, J.; Wong, A.Y.; Ricciardi-Castagnoli, P. Interleukin-2 production by dendritic cells and its immuno-regulatory functions. Front. Immunol. 2012, 3, 161. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Lahrs, S.; Dematteo, R.P. Overexpression of interleukin-12 enables dendritic cells to activate NK cells and confer systemic antitumor immunity. FASEB J. 2003, 17, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Semino, C.; Angelini, G.; Poggi, A.; Rubartelli, A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood 2005, 106, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.L.; Tan, J.K.; O’Neill, H.C. Characterization of the effect of LPS on dendritic cell subset discrimination in spleen. J. Cell. Mol. Med. 2014, 18, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Kurts, C.; Kosaka, H.; Carbone, F.R.; Miller, J.F.; Heath, W.R. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J. Exp. Med. 1997, 186, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; O’Neill, H.C. Maturation requirements for dendritic cells in T cell stimulation leading to tolerance versus immunity. J. Leukoc. Biol. 2005, 78, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Le Bouteiller, P.; Barakonyi, A.; Giustiniani, J.; Lenfant, F.; Marie-Cardine, A.; Aguerre-Girr, M.; Rabot, M.; Hilgert, I.; Mami-Chouaib, F.; Tabiasco, J.; et al. Engagement of CD160 receptor by HLA-C is a triggering mechanism used by circulating natural killer (NK) cells to mediate cytotoxicity. Proc. Natl. Acad. Sci. USA 2002, 99, 16963–16968. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.B.; Hayakawa, Y.; Zerafa, N.; Sheehan, K.C.; Scott, B.; Schreiber, R.D.; Hertzog, P.; Smyth, M.J. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J. Immunol. 2007, 178, 7540–7549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X.; Yang, Y. A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Coccia, E.M.; Battistini, A. Early IFN type I response: Larning from microbial evasion strategies. Semin. Immunol. 2015, 27, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Stifter, S.A.; Feng, C.G. Interfering with immunity: Detrimental role of type I IFNs during infection. J. Immunol. 2015, 194, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Asselin-Paturel, C.; Brizard, G.; Chemin, K.; Boonstra, A.; O’Garra, A.; Vicari, A.; Trinchieri, G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 2005, 201, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Reimer, T.; Brcic, M.; Schweizer, M.; Jungi, T.W. Poly(I:C) and LPS induce distinct IRF3 and NF-kappaB signaling during type-I IFN and TNF responses in human macrophages. J. Leukoc. Biol. 2008, 83, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Vadiveloo, P.K.; Vairo, G.; Hertzog, P.; Kola, I.; Hamilton, J.A. Role of type I interferons during macrophage activation by lipopolysaccharide. Cytokine 2000, 12, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cho, S.Y.; Xiang, G.; Min, K.J.; Yu, Q.; Jin, J.O. Ginseng Berry Extract Promotes Maturation of Mouse Dendritic Cells. PLoS ONE 2015, 10, e0130926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Okimura, T.; Xu, L.; Zhang, L.; Oda, T.; Kwak, M.; Yu, Q.; Jin, J.O. Ascophyllan functions as an adjuvant to promote anti-cancer effect by dendritic cell activation. Oncotarget 2016, 7, 19284–19298. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Han, X.; Yu, Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J. Autoimmun. 2013, 40, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, W.; Xu, L.; Jin, J.-O. Porphyromonas gingivalis Lipopolysaccharide Induced Proliferation and Activation of Natural Killer Cells in Vivo. Molecules 2016, 21, 1086. https://doi.org/10.3390/molecules21081086
Wang Y, Zhang W, Xu L, Jin J-O. Porphyromonas gingivalis Lipopolysaccharide Induced Proliferation and Activation of Natural Killer Cells in Vivo. Molecules. 2016; 21(8):1086. https://doi.org/10.3390/molecules21081086
Chicago/Turabian StyleWang, Yuhua, Wei Zhang, Li Xu, and Jun-O Jin. 2016. "Porphyromonas gingivalis Lipopolysaccharide Induced Proliferation and Activation of Natural Killer Cells in Vivo" Molecules 21, no. 8: 1086. https://doi.org/10.3390/molecules21081086
APA StyleWang, Y., Zhang, W., Xu, L., & Jin, J. -O. (2016). Porphyromonas gingivalis Lipopolysaccharide Induced Proliferation and Activation of Natural Killer Cells in Vivo. Molecules, 21(8), 1086. https://doi.org/10.3390/molecules21081086