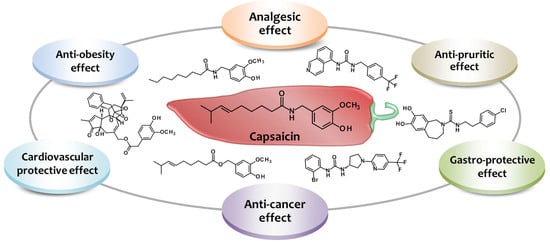
Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases
Abstract
:1. Introduction and Historical Viewpoint
2. Source, Structure, and Analogues of Capsaicin
3. Capsaicin-Induced TRPV1 Signaling, Desensitization and Sensitivity
4. Capsaicin-Derived Agonists and Antagonists
5. Pharmacological Actions of Capsaicin: Implications for Pain Control and More
5.1. Capsaicin-Based Therapies for Management of Chronic Pain
5.1.1. Capsaicin and Neuropathic Pain
5.1.2. Capsaicin and Musculoskeletal Pain
5.1.3. Capsaicin and Migraine
5.2. Anti-cancer Effects of Capsaicin
5.3. Anti-Obesity Effects of Capsaicin
5.4. Role of Capsaicin in the Gastrointestinal System
5.5. Role of Capsaicin in Cardiovascular Diseases
5.6. Role of Capsaicin in Dermatological Disorders
6. On-Target Adverse Effects of Capsaicin
7. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Nunn, N.; Qian, N. The columbian exchange: A history of disease, food, and ideas. J. Econ. Perspect. 2010, 24, 163–188. [Google Scholar] [CrossRef]
- Mozsik, G.; Past, T.; Abdel Salam, O.M.; Kuzma, M.; Perjesi, P. Interdisciplinary review for correlation between the plant origin capsaicinoids, non-steroidal antiinflammatory drugs, gastrointestinal mucosal damage and prevention in animals and human beings. Inflammopharmacology 2009, 17, 113–150. [Google Scholar] [CrossRef] [PubMed]
- Szolcsanyi, J. Capsaicin and sensory neurones: A historical perspective. Prog. Drug Res. 2014, 68, 1–37. [Google Scholar] [PubMed]
- Bucholz, C.F. Chemical investigation of dry, ripe spanish peppers. Alm. Pocket-Book Anal. (Chem.) Apoth. 1816, 37, 1–30. (In German) [Google Scholar]
- Thresh, J.C. Isolation of capsaicin. Pharm. J. Trans. 1876, 6, 941–947. [Google Scholar]
- Micko, K. On our knowledge of capsaicin. J. Inves. Necess. Lux. 1898, 1, 818–829. (In German) [Google Scholar]
- Nelson, E.K. The constitution of capsaicin, the pungent principle of capsicum. J. Am. Chem. Soc. 1919, 41, 1115–1121. [Google Scholar] [CrossRef]
- Spath, S.; Darling, S.F. Synthese des capsaicins. Chem. Ber. 1930, 63B, 737–743. [Google Scholar] [CrossRef]
- Kosuge, S.; Inagaki, Y.; Okumura, H. Studies on the pungent principles of red pepper. Part VIII. On the chemical constitutions of the pungent principles. Nippon NogeiKagaku Kaishi (J. Agric. Chem. Soc. Jpn.) 1961, 35, 923–927. (In Japanese) [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Manchego, C.; Haak, D.C.; Levey, D.J. Where did the chili get its spice? Biogeography of capsaicinoid production in ancestral wild chili species. J. Chem. Ecol. 2006, 32, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C.; Lemos de Andrade, E.; Calixto, J.B. TRP modulation by natural compounds. Handb. Exp. Pharmacol. 2014, 223, 1177–1238. [Google Scholar] [PubMed]
- Buck, S.H.; Burks, T.F. The neuropharmacology of capsaicin: Review of some recent observations. Pharmacol. Rev. 1986, 38, 179–226. [Google Scholar] [PubMed]
- Lee, T.S. Physiological gustatory sweating in a warm climate. J. Physiol. 1954, 124, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Autoradiographic visualization and pharmacological characterization of vanilloid (capsaicin) receptors in several species, including man. Acta Physiol. Scand. Suppl. 1995, 629, 1–68. [Google Scholar] [PubMed]
- Conway, S.J. Trping the switch on pain: An introduction to the chemistry and biology of capsaicin and TRPV1. Chem. Soc. Rev. 2008, 37, 1530–1545. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y.; Olivo, H.F. Chapter 3: Natural and synthetic alkamides: Applications in pain therapy. In Studies in Natural Products Chemistry; Elsevier B.V.: Memphis, TN, USA, 2014; Volume 43, pp. 79–121. [Google Scholar]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A. Tincture of capsaicin as a remedy for chilblains and toothache. Dublin Free Press 1850, 1, 95–96. [Google Scholar]
- Buchheim, R. Fructus capsici. J. Am. Pharm. Assoc. 1873, 22, 106. [Google Scholar]
- Cui, M.; Gosu, V.; Basith, S.; Hong, S.; Choi, S. Polymodal transient receptor potential vanilloid type 1 nocisensor: Structure, modulators, and therapeutic applications. Adv. Protein Chem. Struct. Biol. 2016, 104, 81–125. [Google Scholar] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [PubMed]
- Nagy, I.; Friston, D.; Valente, J.S.; Torres Perez, J.V.; Andreou, A.P. Pharmacology of the capsaicin receptor, transient receptor potential vanilloid type-1 ion channel. Prog. Drug Res. 2014, 68, 39–76. [Google Scholar] [PubMed]
- Qutenza. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000909/human_med_001008.jsp&mid=WC0b01ac058001d124 (accessed on 8 June 2016).
- Haanpaa, M.; Cruccu, G.; Nurmikko, T.J.; McBride, W.T.; Docu Axelarad, A.; Bosilkov, A.; Chambers, C.; Ernault, E.; Abdulahad, A.K. Capsaicin 8% patch versus oral pregabalin in patients with peripheral neuropathic pain. Eur. J. Pain 2016, 20, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Medical Review. 2009; FDA Center for Drug Evaluation and Research. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022395s000sumr.pdf (accessed on 27 November 2015).
- Baranidharan, G.; Das, S.; Bhaskar, A. A review of the high-concentration capsaicin patch and experience in its use in the management of neuropathic pain. Ther. Adv. Neurol. Disord. 2013, 6, 287–297. [Google Scholar] [CrossRef] [PubMed]
- De Lille, J.; Ramirez, E. Pharmacodynamic action of the active principles of chillie. Chem. Abstr. 1935, 29, 4836. [Google Scholar]
- Toh, C.C.; Lee, T.S.; Kiang, A.K. The pharmacological actions of capsaicin and analogues. Br. J. Pharmacol. Chemother. 1955, 10, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Magi, G.; Mingoia, M.; Pugnaloni, A.; Facinelli, B. Antimicrobial and anti-virulence activity of capsaicin against erythromycin-resistant, cell-invasive group a streptococci. Front. Microbiol. 2015, 6, 1281. [Google Scholar] [CrossRef] [PubMed]
- Dorantes, L.; Colmenero, R.; Hernandez, H.; Mota, L.; Jaramillo, M.E.; Fernandez, E.; Solano, C. Inhibition of growth of some foodborne pathogenic bacteria by capsicum annum extracts. Int. J. Food Microbiol. 2000, 57, 125–128. [Google Scholar] [CrossRef]
- Walpole, C.S.; Wrigglesworth, R.; Bevan, S.; Campbell, E.A.; Dray, A.; James, I.F.; Perkins, M.N.; Reid, D.J.; Winter, J. Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 1. The aromatic “a-region”. J. Med. Chem. 1993, 36, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Walpole, C.S.; Bevan, S.; Bloomfield, G.; Breckenridge, R.; James, I.F.; Ritchie, T.; Szallasi, A.; Winter, J.; Wrigglesworth, R. Similarities and differences in the structure-activity relationships of capsaicin and resiniferatoxin analogues. J. Med. Chem. 1996, 39, 2939–2952. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; St-Pierre, S.; Suzuki, M.; Tremblay, A. Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. Br. J. Nutr. 1998, 80, 503–510. [Google Scholar] [PubMed]
- Arabaci, B.; Gulcin, I.; Alwasel, S. Capsaicin: A potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2014, 19, 10103–10114. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; Brock, C.; Olesen, A.E.; Andresen, T.; Nilsson, M.; Dickenson, A.H. Unravelling the mystery of capsaicin: A tool to understand and treat pain. Pharmacol. Rev. 2012, 64, 939–971. [Google Scholar] [CrossRef] [PubMed]
- Al Othman, Z.A.; Ahmed, Y.B.; Habila, M.A.; Ghafar, A.A. Determination of capsaicin and dihydrocapsaicin in capsicum fruit samples using high performance liquid chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.V.; Schreiber, A.A.; Weisskopf, C.P. Simple method for quantitation of capsaicinoids in peppers using capillary gas chromatography. J. Agric. Food Chem. 1998, 46, 2655–2663. [Google Scholar] [CrossRef]
- Cordell, G.A.; Araujo, O.E. Capsaicin: Identification, nomenclature, and pharmacotherapy. Ann. Pharmacother. 1993, 27, 330–336. [Google Scholar] [PubMed]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shintaku, K.; Uchida, K.; Suzuki, Y.; Zhou, Y.; Fushiki, T.; Watanabe, T.; Yazawa, S.; Tominaga, M. Activation of transient receptor potential a1 by a non-pungent capsaicin-like compound, capsiate. Br. J. Pharmacol. 2012, 165, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with aδ/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.; Hargreaves, K.M.; Akopian, A.N. TRPA1-mediated responses in trigeminal sensory neurons: Interaction between TRPA1 and TRPV1. Eur. J. Neurosci. 2009, 29, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, M.Z.P.; Jordt, S.E. TRPA1: A sensory channel of many talents. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ho, K.W.; Ward, N.J.; Calkins, D.J. TRPV1: A stress response protein in the central nervous system. Am. J. Neurodegener. Dis. 2012, 1, 1–14. [Google Scholar] [PubMed]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, X.; Cheng, W.; Yang, W.; Yu, P.; Song, Z.; Yarov-Yarovoy, V.; Zheng, J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 2015, 11, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.; Kumar Mishra, A. Location, partitioning behavior, and interaction of capsaicin with lipid bilayer membrane: Study using its intrinsic fluorescence. J. Phys. Chem. B 2015, 119, 12086–12093. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.J.; Yu, S.Y.; Kim, D.I.; Suh, B.C. Differential regulation of proton-sensitive ion channels by phospholipids: A comparative study between asics and TRPV1. PLoS ONE 2015, 10, e0122014. [Google Scholar] [CrossRef] [PubMed]
- Aneiros, E.; Cao, L.; Papakosta, M.; Stevens, E.B.; Phillips, S.; Grimm, C. The biophysical and molecular basis of TRPV1 proton gating. EMBO J. 2011, 30, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Tominaga, M.; Julius, D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl Acad. Sci. USA 2000, 97, 8134–8139. [Google Scholar] [CrossRef] [PubMed]
- Devesa, I.; Planells-Cases, R.; Fernandez-Ballester, G.; Gonzalez-Ros, J.M.; Ferrer-Montiel, A.; Fernandez-Carvajal, A. Role of the transient receptor potential vanilloid 1 in inflammation and sepsis. J. Inflamm. Res. 2011, 4, 67–81. [Google Scholar] [PubMed]
- Jancso, N.; Jancso-Gabor, A.; Szolcsanyi, J. The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br. J. Pharmacol. Chemother. 1968, 33, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Smutzer, G.; Devassy, R.K. Integrating TRPV1 receptor function with capsaicin psychophysics. Adv. Pharmacol. Sci. 2016, 2016, 1512457. [Google Scholar] [CrossRef] [PubMed]
- Donnerer, J.; Amann, R. The inhibition of neurogenic inflammation. Gen. Pharmacol. 1993, 24, 519–529. [Google Scholar] [CrossRef]
- Shimomura, Y.; Kawada, T.; Suzuki, M. Capsaicin and its analogs inhibit the activity of nadh-coenzyme q oxidoreductase of the mitochondrial respiratory chain. Arch. Biochem. Biophys. 1989, 270, 573–577. [Google Scholar] [CrossRef]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar] [PubMed]
- Docherty, R.J.; Yeats, J.C.; Bevan, S.; Boddeke, H.W. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflug. Arch. 1996, 431, 828–837. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Nau, C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic amp-dependent protein kinase pathway. J. Biol. Chem. 2003, 278, 50080–50090. [Google Scholar] [CrossRef] [PubMed]
- Szolcsanyi, J.; Jancso-Gabor, A. Sensory effects of capsaicin congeners I. Relationship between chemical structure and pain-producing potency of pungent agents. Arzneimittelforschung 1975, 25, 1877–1881. [Google Scholar] [PubMed]
- Szolcsanyi, J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J. Physiol. (Paris) 1977, 73, 251–259. [Google Scholar]
- Nolano, M.; Simone, D.A.; Wendelschafer-Crabb, G.; Johnson, T.; Hazen, E.; Kennedy, W.R. Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain 1999, 81, 135–145. [Google Scholar] [CrossRef]
- Simone, D.A.; Nolano, M.; Johnson, T.; Wendelschafer-Crabb, G.; Kennedy, W.R. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: Correlation with sensory function. J. Neurosci. 1998, 18, 8947–8959. [Google Scholar] [PubMed]
- Szolcsanyi, J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004, 38, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Hail, N., Jr. Mechanisms of vanilloid-induced apoptosis. Apoptosis 2003, 8, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Santha, P.; Jancso, G. Transganglionic transport of choleragenoid by capsaicin-sensitive c-fibre afferents to the substantia gelatinosa of the spinal dorsal horn after peripheral nerve section. Neuroscience 2003, 116, 621–627. [Google Scholar] [CrossRef]
- Jancso, G.; Dux, M.; Oszlacs, O.; Santha, P. Activation of the transient receptor potential vanilloid-1 (TRPV1) channel opens the gate for pain relief. Br. J. Pharmacol. 2008, 155, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Tewksbury, J.J.; Nabhan, G.P. Seed dispersal. Directed deterrence by capsaicin in chilies. Nature 2001, 412, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Tewksbury, J.J.; Reagan, K.M.; Machnicki, N.J.; Carlo, T.A.; Haak, D.C.; Penaloza, A.L.; Levey, D.J. Evolutionary ecology of pungency in wild chilies. Proc. Natl. Acad. Sci. USA 2008, 105, 11808–11811. [Google Scholar] [CrossRef] [PubMed]
- Levey, D.J.; Tewksbury, J.J.; Cipollini, M.L.; Carlo, T.A. A field test of the directed deterrence hypothesis in two species of wild chili. Oecologia 2006, 150, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J.; Barthó, L. Impaired defense mechanisms to peptic ulcer in the capsaicin desensitized rat. In Advances in Physiological Sciences, Gastrointestinal Defense Mechanisms; Mózsik, G.Y., Hänninen, O., Jávor, T., Eds.; Ergamon Press: Budapest, Hungary, 1981; Volume 29, pp. 39–51. [Google Scholar]
- Jessell, T.M.; Iversen, L.L.; Cuello, A.C. Capsaicin-induced depletion of substance p from primary sensory neurones. Brain Res. 1978, 152, 183–188. [Google Scholar] [CrossRef]
- Jancso, G.; Kiraly, E.; Jancso-Gabor, A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 1977, 270, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Iversen, L.L.; Goedert, M.; Chapman, D.; Hunt, S.P. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J. Neurosci. 1983, 3, 399–406. [Google Scholar] [PubMed]
- Kissin, I.; Szallasi, A. Therapeutic targeting of TRPV1 by resiniferatoxin, from preclinical studies to clinical trials. Curr. Top. Med. Chem. 2011, 11, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Salat, K.; Jakubowska, A.; Kulig, K. Zucapsaicin for the treatment of neuropathic pain. Expert Opin. Investig. Drugs 2014, 23, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Bevan, S.; Hothi, S.; Hughes, G.; James, I.F.; Rang, H.P.; Shah, K.; Walpole, C.S.; Yeats, J.C. Capsazepine: A competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992, 107, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Trbovich, M.; Yang, H. Capsaicin 8% patch for central and peripheral neuropathic pain of persons with incomplete spinal cord injury: Two case reports. Am. J. Phys. Med. Rehabil. 2015, 94, e66–e72. [Google Scholar] [CrossRef] [PubMed]
- Remadevi, R.; Szallisi, A. Adlea (ALGRX-4975), an injectable capsaicin (TRPV1 receptor agonist) formulation for longlasting pain relief. IDrugs 2008, 11, 120–132. [Google Scholar] [PubMed]
- Iadarola, M.J.; Gonnella, G.L. Resiniferatoxin for pain treatment: An interventional approach to personalized pain medicine. Open Pain J. 2013, 6, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Gaubitz, M.; Schiffer, T.; Holm, C.; Richter, E.; Pisternick-Ruf, W.; Weiser, T. Efficacy and safety of nicoboxil/nonivamide ointment for the treatment of acute pain in the low back—A randomized, controlled trial. Eur. J. Pain 2016, 20, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Snitker, S.; Fujishima, Y.; Shen, H.; Ott, S.; Pi-Sunyer, X.; Furuhata, Y.; Sato, H.; Takahashi, M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: Possible pharmacogenetic implications. Am. J. Clin. Nutr. 2009, 89, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.M.; Urban, L.; Medhurst, S.J.; Patel, S.; Panesar, M.; Fox, A.J.; McIntyre, P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2003, 304, 56–62. [Google Scholar] [CrossRef] [PubMed]
- El Kouhen, R.; Surowy, C.S.; Bianchi, B.R.; Neelands, T.R.; McDonald, H.A.; Niforatos, W.; Gomtsyan, A.; Lee, C.H.; Honore, P.; Sullivan, J.P.; et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel and selective transient receptor potential type V1 receptor antagonist, blocks channel activation by vanilloids, heat, and acid. J. Pharmacol. Exp. Ther. 2005, 314, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Gavva, N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.A.; Nothaft, W.; Awni, W.M.; Dutta, S. Effects of the TRPV1 antagonist ABT-102 on body temperature in healthy volunteers: Pharmacokinetic/pharmacodynamic analysis of three phase 1 trials. Br. J. Clin. Pharmacol. 2013, 75, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Jts 653. Available online: http://adisinsight.springer.com/drugs/800028532 (accessed on 7 March 2016).
- Japic Clinical Trials Information. Available online: http://www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-101177 (accessed on 7 March 2016).
- Smith, H.; Brooks, J.R. Capsaicin-based therapies for pain control. Prog. Drug Res. 2014, 68, 129–146. [Google Scholar] [PubMed]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B. Trp channels in disease. Biochim. Biophys. Acta 2007, 1772, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Immke, D.C.; Gavva, N.R. The TRPV1 receptor and nociception. Semin. Cell Dev. Biol. 2006, 17, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, S. Novel therapeutics in the field of capsaicin and pain. Expert Rev. Clin. Pharmacol. 2015, 8, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Audette, J.; Baron, R.; Gourlay, G.K.; Haanpaa, M.L.; Kent, J.L.; Krane, E.J.; Lebel, A.A.; Levy, R.M.; et al. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clin. Proc. 2010, 85, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Moore, R.A. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2012, 12. [Google Scholar] [CrossRef]
- Casanueva, B.; Rodero, B.; Quintial, C.; Llorca, J.; Gonzalez-Gay, M.A. Short-term efficacy of topical capsaicin therapy in severely affected fibromyalgia patients. Rheumatol. Int. 2013, 33, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Robbins, W.R.; Staats, P.S.; Levine, J.; Fields, H.L.; Allen, R.W.; Campbell, J.N.; Pappagallo, M. Treatment of intractable pain with topical large-dose capsaicin: Preliminary report. Anesth. Analg. 1998, 86, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Sven-Rice, A.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2013, 2, CD007393. [Google Scholar] [PubMed]
- Treede, R.D.; Wagner, T.; Kern, K.U.; Husstedt, I.W.; Arendt, G.; Birklein, F.; Cegla, T.; Freynhagen, R.; Gockel, H.H.; Heskamp, M.L.; et al. Mechanism- and experience-based strategies to optimize treatment response to the capsaicin 8% cutaneous patch in patients with localized neuropathic pain. Curr. Med. Res. Opin. 2013, 29, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, W.R.; Vanhove, G.F.; Lu, S.P.; Tobias, J.; Bley, K.R.; Walk, D.; Wendelschafer-Crabb, G.; Simone, D.A.; Selim, M.M. A randomized, controlled, open-label study of the long-term effects of ngx-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J. Pain 2010, 11, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Backonja, M.; Wallace, M.S.; Blonsky, E.R.; Cutler, B.J.; Malan, P., Jr.; Rauck, R.; Tobias, J. Ngx-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: A randomised, double-blind study. Lancet Neurol. 2008, 7, 1106–1112. [Google Scholar] [CrossRef]
- Irving, G.A.; Backonja, M.M.; Dunteman, E.; Blonsky, E.R.; Vanhove, G.F.; Lu, S.P.; Tobias, J. A multicenter, randomized, double-blind, controlled study of ngx-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med. 2011, 12, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R.; Peppin, J.F.; Murphy, F.T.; Tobias, J.K.; Vanhove, G.F. Tolerability of ngx-4010, a capsaicin 8% patch, in conjunction with three topical anesthetic formulations for the treatment of neuropathic pain. J. Pain Res. 2012, 5, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R.; Nunez, M.; Tark, M.D.; Dunteman, E.D.; Lu, B.; Tobias, J.K.; Vanhove, G.F. Tolerability of ngx-4010, a capsaicin 8% dermal patch, following pretreatment with lidocaine 2.5%/prilocaine 2.5% cream in patients with post-herpetic neuralgia. BMC Anesthesiol. 2011, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.; Moore, R.A.; Derry, S.; Edwards, J.E.; McQuay, H.J. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ 2004, 328, 991. [Google Scholar] [CrossRef] [PubMed]
- Laslett, L.L.; Jones, G. Capsaicin for osteoarthritis pain. Prog. Drug Res. 2014, 68, 277–291. [Google Scholar] [PubMed]
- Deal, C.L.; Schnitzer, T.J.; Lipstein, E.; Seibold, J.R.; Stevens, R.M.; Levy, M.D.; Albert, D.; Renold, F. Treatment of arthritis with topical capsaicin: A double-blind trial. Clin. Ther. 1991, 13, 383–395. [Google Scholar] [PubMed]
- McCarthy, G.M.; McCarty, D.J. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J. Rheumatol. 1992, 19, 604–607. [Google Scholar] [PubMed]
- Altman, R.D.; Aven, A.; Holmburg, C.E.; Pfeifer, L.M.; Sack, M.; Young, G.T. Capsaicin cream 0.025% as monotherapy for osteoarthritis: A double-blind study. Semin. Arthritis Rheum. 1994, 23, 25–33. [Google Scholar] [CrossRef]
- McCleane, G. The analgesic efficacy of topical capsaicin is enhanced by glyceryl trinitrate in painful osteoarthritis: A randomized, double blind, placebo controlled study. Eur. J. Pain 2000, 4, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, T.J.; Pelletier, J.P.; Haselwood, D.M.; Ellison, W.T.; Ervin, J.E.; Gordon, R.D.; Lisse, J.R.; Archambault, W.T.; Sampson, A.R.; Fezatte, H.B.; et al. Civamide cream 0.075% in patients with osteoarthritis of the knee: A 12-week randomized controlled clinical trial with a longterm extension. J. Rheumatol. 2012, 39, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Kosuwon, W.; Sirichatiwapee, W.; Wisanuyotin, T.; Jeeravipoolvarn, P.; Laupattarakasem, W. Efficacy of symptomatic control of knee osteoarthritis with 0.0125% of capsaicin versus placebo. J. Med. Assoc. Thail. 2010, 93, 1188–1195. [Google Scholar]
- Schnitzer, T.J.; Posner, M.; Lawrence, I.D. High strength capsaicin cream for osteoarthritis pain: Rapid onset of action and improved efficacy with twice daily dosing. J. Clin. Rheumatol. 1995, 1, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthrits Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef]
- Mathew, N.T.; Reuveni, U.; Perez, F. Transformed or evolutive migraine. Headache 1987, 27, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Fusco, B.M.; Barzoi, G.; Agro, F. Repeated intranasal capsaicin applications to treat chronic migraine. Br. J. Anaesth. 2003, 90, 812. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.R.; Rapoport, A.; Padla, D.; Weeks, R.; Rosum, R.; Sheftell, F.; Arrowsmith, F. A double-blind placebo-controlled trial of intranasal capsaicin for cluster headache. Cephalalgia 1993, 13, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Fusco, B.M.; Marabini, S.; Maggi, C.A.; Fiore, G.; Geppetti, P. Preventative effect of repeated nasal applications of capsaicin in cluster headache. Pain 1994, 59, 321–325. [Google Scholar] [CrossRef]
- Diamond, S.; Freitag, F.; Phillips, S.B.; Bernstein, J.E.; Saper, J.R. Intranasal civamide for the acute treatment of migraine headache. Cephalalgia 2000, 20, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Saper, J.R.; Klapper, J.; Mathew, N.T.; Rapoport, A.; Phillips, S.B.; Bernstein, J.E. Intranasal civamide for the treatment of episodic cluster headaches. Arch. Neurol. 2002, 59, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Cianchetti, C. Capsaicin jelly against migraine pain. Int. J. Clin. Pract. 2010, 64, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Laviada, I.; Rodriguez-Henche, N. The potential antitumor effects of capsaicin. Prog. Drug Res. 2014, 68, 181–208. [Google Scholar] [PubMed]
- Lin, C.H.; Lu, W.C.; Wang, C.W.; Chan, Y.C.; Chen, M.K. Capsaicin induces cell cycle arrest and apoptosis in human kb cancer cells. BMC Complement. Altern. Med. 2013, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.; Calzado, M.A.; Munoz-Blanco, J.; Gomez-Diaz, C.; Gajate, C.; Mollinedo, F.; Navas, P.; Munoz, E. Selective induction of apoptosis by capsaicin in transformed cells: The role of reactive oxygen species and calcium. Cell Death Differ. 1999, 6, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Srivastava, S.K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ros generation and mitochondrial death pathway. Apoptosis 2008, 13, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Boreddy, S.R.; Srivastava, S.K. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS ONE 2011, 6, e20151. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.K.; Tavares, M.T.; Pasqualoto, K.F.; de Azevedo, R.A.; Teixeira, S.F.; Ferreira-Junior, W.A.; Bertin, A.M.; de-Sa-Junior, P.L.; Barbuto, J.A.; Figueiredo, C.R.; et al. Rpf151, a novel capsaicin-like analogue: In vitro studies and in vivo preclinical antitumor evaluation in a breast cancer model. Tumour Biol. 2015, 36, 7251–7267. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, J.; Lee, S.H. Synergistic anticancer activity of capsaicin and 3,3’-diindolylmethane in human colorectal cancer. J. Agric. Food Chem. 2015, 63, 4297–4304. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Bhattacharjee, S.; Mandal, D.P. Induction of apoptosis by eugenol and capsaicin in human gastric cancer ags cells—Elucidating the role of p53. Asian Pac. J. Cancer Prev. 2015, 16, 6753–6759. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.Q.; Cai, K.; Shen, F.; Bao, X.D.; Xu, Y.; Yu, F.; Pan, H.Q.; Chen, C.H.; Du, Z.J.; Cui, J.H. Induction of apoptosis by capsaicin in hepatocellular cancer cell line smmc-7721 is mediated through ros generation and activation of jnk and p38 mapk pathways. Neoplasma 2015, 62, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Chen, S.T.; Chien, S.Y.; Kuo, S.J.; Tsai, H.T.; Chen, D.R. Capsaicin may induce breast cancer cell death through apoptosis-inducing factor involving mitochondrial dysfunction. Hum. Exp. Toxicol. 2011, 30, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Wu, Y.C.; Wang, Y.F.; Chou, M.J.; Kuo, S.J.; Chen, D.R. Capsaicin-induced apoptosis in human breast cancer mcf-7 cells through caspase-independent pathway. Oncol. Rep. 2009, 21, 665–671. [Google Scholar] [PubMed]
- Thoennissen, N.H.; O’Kelly, J.; Lu, D.; Iwanski, G.B.; La, D.T.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H.P. Capsaicin causes cell-cycle arrest and apoptosis in er-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Jung, Y.K.; Oh, S.H. Autophagy induction by capsaicin in malignant human breast cells is modulated by p38 and extracellular signal-regulated mitogen-activated protein kinases and retards cell death by suppressing endoplasmic reticulum stress-mediated apoptosis. Mol. Pharmacol. 2010, 78, 114–125. [Google Scholar] [CrossRef] [PubMed]
- De-Sa-Junior, P.L.; Pasqualoto, K.F.; Ferreira, A.K.; Tavares, M.T.; Damiao, M.C.; de Azevedo, R.A.; Camara, D.A.; Pereira, A.; de Souza, D.M.; Parise Filho, R. Rpf101, a new capsaicin-like analogue, disrupts the microtubule network accompanied by arrest in the G2/M phase, inducing apoptosis and mitotic catastrophe in the mcf-7 breast cancer cells. Toxicol. Appl. Pharmacol. 2013, 266, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Wutka, A.; Palagani, V.; Barat, S.; Chen, X.; El Khatib, M.; Gotze, J.; Belahmer, H.; Zender, S.; Bozko, P.; Malek, N.P.; et al. Capsaicin treatment attenuates cholangiocarcinoma carcinogenesis. PLoS ONE 2014, 9, e95605. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin represses transcriptional activity of beta-catenin in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, T.Z.; Xu, G.H.; Luo, B.B.; Chen, Y.X.; Zhang, T. Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ros production and modulating AKT/mTOR and stat-3 pathways. Neoplasma 2013, 60, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Lim, S.C. Endoplasmic reticulum stress-mediated autophagy/apoptosis induced by capsaicin (8-methyl-n-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal-regulated kinase activation in wi38 lung epithelial fibroblast cells. J. Pharmacol. Exp. Ther. 2009, 329, 112–122. [Google Scholar] [PubMed]
- Kim, Y.M.; Hwang, J.T.; Kwak, D.W.; Lee, Y.K.; Park, O.J. Involvement of ampk signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann. N. Y. Acad. Sci. 2007, 1095, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Chen, Y.L.; Yang, J.S.; Yang, Y.Y.; Liu, J.Y.; Hsu, S.C.; Lai, K.C.; Chung, J.G. Antitumor activity of capsaicin on human colon cancer cells in vitro and colo 205 tumor xenografts in vivo. J. Agric. Food Chem. 2010, 58, 12999–13005. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Yang, Y.C.; Wu, I.C.; Kuo, F.C.; Liu, C.M.; Wang, H.W.; Kuo, C.H.; Wu, J.Y.; Wu, D.C. Capsaicin-induced cell death in a human gastric adenocarcinoma cell line. World J. Gastroenterol. 2005, 11, 6254–6257. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.Y.; Lee, S.M.; Jun, C.H.; Cho, S.B.; Park, C.H.; Joo, Y.E.; Kim, H.S.; Choi, S.K.; Rew, J.S. Capsaicin induces apoptosis and modulates mapk signaling in human gastric cancer cells. Mol. Med. Rep. 2014, 9, 499–502. [Google Scholar] [PubMed]
- Huh, H.C.; Lee, S.Y.; Lee, S.K.; Park, N.H.; Han, I.S. Capsaicin induces apoptosis of cisplatin-resistant stomach cancer cells by causing degradation of cisplatin-inducible aurora-a protein. Nutr. Cancer 2011, 63, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Meral, O.; Alpay, M.; Kismali, G.; Kosova, F.; Cakir, D.U.; Pekcan, M.; Yigit, S.; Sel, T. Capsaicin inhibits cell proliferation by cytochrome C release in gastric cancer cells. Tumour Biol. 2014, 35, 6485–6492. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.P.; Chen, J.C.; Wu, C.C.; Chen, C.T.; Tang, N.Y.; Ho, Y.T.; Lo, C.; Lin, J.P.; Chung, J.G.; Lin, J.G. Capsaicin-induced apoptosis in human hepatoma hepg2 cells. Anticancer Res. 2009, 29, 165–174. [Google Scholar] [PubMed]
- Moon, D.O.; Kang, C.H.; Kang, S.H.; Choi, Y.H.; Hyun, J.W.; Chang, W.Y.; Kang, H.K.; Koh, Y.S.; Maeng, Y.H.; Kim, Y.R.; et al. Capsaicin sensitizes trail-induced apoptosis through sp1-mediated DR5 up-regulation: Involvement of Ca2+ influx. Toxicol. Appl. Pharmacol. 2012, 259, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Lai, F.J.; Chen, H.; Luo, J.; Zhang, R.Y.; Bu, H.Q.; Wang, Z.H.; Lin, H.H.; Lin, S.Z. Involvement of the phosphoinositide 3-kinase/AKT pathway in apoptosis induced by capsaicin in the human pancreatic cancer cell line panc-1. Oncol. Lett. 2013, 5, 43–48. [Google Scholar] [PubMed]
- Sanchez, A.M.; Sanchez, M.G.; Malagarie-Cazenave, S.; Olea, N.; Diaz-Laviada, I. Induction of apoptosis in prostate tumor pc-3 cells and inhibition of xenograft prostate tumor growth by the vanilloid capsaicin. Apoptosis 2006, 11, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Malagarie-Cazenave, S.; Olea, N.; Vara, D.; Chiloeches, A.; Diaz-Laviada, I. Apoptosis induced by capsaicin in prostate pc-3 cells involves ceramide accumulation, neutral sphingomyelinase, and jnk activation. Apoptosis 2007, 12, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Y.C.; Lu, W.H.; Huang, P.; Hu, Y. Selective killing of k-ras-transformed pancreatic cancer cells by targeting NAD(P)H oxidase. Chin. J. Cancer 2015, 34, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Fofaria, N.M.; Gupta, P.; Ranjan, A.; Kim, S.H.; Srivastava, S.K. Inhibition of beta-catenin signaling suppresses pancreatic tumor growth by disrupting nuclear β-catenin/tcf-1 complex: Critical role of stat-3. Oncotarget 2015, 6, 11561–11574. [Google Scholar] [CrossRef] [PubMed]
- Venier, N.A.; Colquhoun, A.J.; Sasaki, H.; Kiss, A.; Sugar, L.; Adomat, H.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Capsaicin: A novel radio-sensitizing agent for prostate cancer. Prostate 2015, 75, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Venier, N.A.; Yamamoto, T.; Sugar, L.M.; Adomat, H.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Capsaicin reduces the metastatic burden in the transgenic adenocarcinoma of the mouse prostate model. Prostate 2015, 75, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, J.; Ma, Z.; Liu, W.; Yang, F.; Yang, Z.; Wang, K.; Wang, X.; He, D.; Li, L. Capsaicin causes inactivation and degradation of the androgen receptor by inducing the restoration of mir-449a in prostate cancer. Oncol. Rep. 2015, 34, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Whiting, S.; Derbyshire, E.; Tiwari, B.K. Capsaicinoids and capsinoids. A potential role for weight management? A systematic review of the evidence. Appetite 2012, 59, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.; Derbyshire, E.J.; Tiwari, B. Could capsaicinoids help to support weight management? A systematic review and meta-analysis of energy intake data. Appetite 2014, 73, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Belza, A.; Frandsen, E.; Kondrup, J. Body fat loss achieved by stimulation of thermogenesis by a combination of bioactive food ingredients: A placebo-controlled, double-blind 8-week intervention in obese subjects. Int. J. Obes. (Lond.) 2007, 31, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Yan Liu, D.; Ma, L.Q.; Luo, Z.D.; Cao, T.B.; Zhong, J.; Yan, Z.C.; Wang, L.J.; Zhao, Z.G.; Zhu, S.J.; et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 2007, 100, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Tsuyoshi, G.; Le Ngoc, H.; Kim, H.M.; Tu, T.H.; Noh, H.J.; Kim, C.S.; Choe, S.Y.; Kawada, T.; Yoo, H.; et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J. Med. Food 2011, 14, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Goto, T.; Han, I.S.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity (Silver Spring) 2010, 18, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Garami, A.; Balasko, M.; Szekely, M.; Solymar, M.; Petervari, E. Fasting hypometabolism and refeeding hyperphagia in rats: Effects of capsaicin desensitization of the abdominal vagus. Eur. J. Pharmacol. 2010, 644, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.I.; Kim, D.H.; Choi, J.W.; Yun, J.W. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Stearns, A.T.; Balakrishnan, A.; Radmanesh, A.; Ashley, S.W.; Rhoads, D.B.; Tavakkolizadeh, A. Relative contributions of afferent vagal fibers to resistance to diet-induced obesity. Dig. Dis. Sci. 2012, 57, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yoneshiro, T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr. Opin. Lipidol. 2013, 24, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J. Agric. Food Chem. 2007, 55, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Lim, K.; Kikuzato, S.; Kiyonaga, A.; Tanaka, H.; Shindo, M.; Suzuki, M. Effects of red-pepper diet on the energy metabolism in men. J. Nutr. Sci. Vitaminol. (Tokyo) 1995, 41, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; St-Pierre, S.; Drapeau, V.; Dionne, I.; Doucet, E.; Suzuki, M.; Tremblay, A. Effects of red pepper on appetite and energy intake. Br. J. Nutr. 1999, 82, 115–123. [Google Scholar] [PubMed]
- Yoshioka, M.; Doucet, E.; Drapeau, V.; Dionne, I.; Tremblay, A. Combined effects of red pepper and caffeine consumption on 24 h energy balance in subjects given free access to foods. Br. J. Nutr. 2001, 85, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.S.; Smeets, A.; Lejeune, M.P. Sensory and gastrointestinal satiety effects of capsaicin on food intake. Int J. Obes. (Lond.) 2005, 29, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.W. Capsaicin as an anti-obesity drug. Prog. Drug Res. 2014, 68, 171–179. [Google Scholar] [PubMed]
- Mozsik, G. Capsaicin as new orally applicable gastroprotective and therapeutic drug alone or in combination with nonsteroidal anti-inflammatory drugs in healthy human subjects and in patients. Prog. Drug Res. 2014, 68, 209–258. [Google Scholar] [PubMed]
- Mózsik, G.; Past, T.; Habon, T.; Keszthelyi, Z.; Perjési, P.; Kuzma, M.; Sándor, B.; Szolcsányi, J.; Abdel-Salam Omar, M.E.; Szalai, M. Chapter 11: Capsaicin is a new gastrointestinal mucosal protecting drug candidate in humans—Pharmaceutical development and production based on clinical pharmacology. In Capsaicin—Sensitive Neural Afferentation and the Gastrointestinal Tract: From Bench to Bedside; Mozsik, G., Abdel-Salam Omar, M.E., Takeuchi, K., Eds.; InTech: Rijeka, Croatia, 2014; pp. 265–364. [Google Scholar]
- Bortolotti, M.; Coccia, G.; Grossi, G.; Miglioli, M. The treatment of functional dyspepsia with red pepper. Aliment. Pharmacol. Ther. 2002, 16, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, M.; Hammer, J. Effect of repeated, long term capsaicin ingestion on intestinal chemo- and mechanosensation in healthy volunteers. Neurogastroenterol. Motil. 2009, 21, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, K.G.; Kang, J.Y.; Yap, I.; Guan, R.; Tan, C.C.; Wee, A.; Teng, C.H. Chili protects against aspirin-induced gastroduodenal mucosal injury in humans. Dig. Dis. Sci. 1995, 40, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Porta, S. Effect of red pepper on symptoms of irritable bowel syndrome: Preliminary study. Dig. Dis. Sci. 2011, 56, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Wang, F.; Chen, J.; Zhao, Y.; Wang, P.; Nilius, B.; Liu, D.; Zhu, Z. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor delta activation. Pflug. Arch. 2013, 465, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Mozsik, G.; Szolcsanyi, J.; Racz, I. Gastroprotection induced by capsaicin in healthy human subjects. World J. Gastroenterol. 2005, 11, 5180–5184. [Google Scholar] [PubMed]
- Prakash, U.N.; Srinivasan, K. Beneficial influence of dietary spices on the ultrastructure and fluidity of the intestinal brush border in rats. Br. J. Nutr. 2010, 104, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Prakash, U.N.; Srinivasan, K. Enhanced intestinal uptake of iron, zinc and calcium in rats fed pungent spice principles—Piperine, capsaicin and ginger (zingiber officinale). J. Trace Elem. Med. Biol. 2013, 27, 184–190. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart 2015, 2, e000262. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Ball, M.J. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. Br. J. Nutr. 2006, 96, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, D.M.; LaHann, T.R. Effects of capsaicinoids on platelet aggregation. Proc. West. Pharmacol. Soc. 1989, 32, 95–100. [Google Scholar] [PubMed]
- Meddings, J.B.; Hogaboam, C.M.; Tran, K.; Reynolds, J.D.; Wallace, J.L. Capsaicin effects on non-neuronal plasma membranes. Biochim. Biophys. Acta 1991, 1070, 43–50. [Google Scholar] [CrossRef]
- Mittelstadt, S.W.; Nelson, R.A.; Daanen, J.F.; King, A.J.; Kort, M.E.; Kym, P.R.; Lubbers, N.L.; Cox, B.F.; Lynch, J.J., III. Capsaicin-induced inhibition of platelet aggregation is not mediated by transient receptor potential vanilloid type 1. Blood Coagul. Fibrinolysis 2012, 23, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.G.; Brownlow, S.L.; Sage, S.O. A role for TRPV1 in agonist-evoked activation of human platelets. J. Thromb. Haemost. 2009, 7, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Zvara, A.; Bencsik, P.; Fodor, G.; Csont, T.; Hackler, L., Jr.; Dux, M.; Furst, S.; Jancso, G.; Puskas, L.G.; Ferdinandy, P. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: A DNA microarray study. FASEB J. 2006, 20, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Y.J. The vanilloid receptor TRPV1: Role in cardiovascular and gastrointestinal protection. Eur. J. Pharmacol. 2010, 627, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Hsiao, H.C.; Wu, D.C.; Lin, R.J.; Liang, J.C.; Yeh, J.L.; Chen, I.J. A novel capsaicin derivative voa induced relaxation in rat mesenteric and aortic arteries: Involvement of cgrp, no, cgmp, and endothelium-dependent activities. J. Cardiovasc. Pharmacol. 2003, 42, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Bratz, I.N.; Dick, G.M.; Tune, J.D.; Edwards, J.M.; Neeb, Z.P.; Dincer, U.D.; Sturek, M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2489–H2496. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, H.; Zhang, Y.; Wang, L.; Zheng, L. Vasodilating effect of capsaicin on rat mesenteric artery and its mechanism. Zhejiang Da Xue Xue Bao Yi Xue Ban 2013, 42, 177–183. (In Chinese) [Google Scholar] [PubMed]
- Xu, X.; Wang, P.; Zhao, Z.; Cao, T.; He, H.; Luo, Z.; Zhong, J.; Gao, F.; Zhu, Z.; Li, L.; et al. Activation of transient receptor potential vanilloid 1 by dietary capsaicin delays the onset of stroke in stroke-prone spontaneously hypertensive rats. Stroke 2011, 42, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Fragasso, G.; Palloshi, A.; Piatti, P.M.; Monti, L.; Rossetti, E.; Setola, E.; Montano, C.; Bassanelli, G.; Calori, G.; Margonato, A. Nitric-oxide mediated effects of transdermal capsaicin patches on the ischemic threshold in patients with stable coronary disease. J. Cardiovasc. Pharmacol. 2004, 44, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.S.; Tak, M.H.; Lee, M.H.; Kim, M.; Koo, J.Y.; Lee, C.H.; Oh, U. TRPV1 mediates histamine-induced itching via the activation of phospholipase a2 and 12-lipoxygenase. J. Neurosci. 2007, 27, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Imamachi, N.; Park, G.H.; Lee, H.; Anderson, D.J.; Simon, M.I.; Basbaum, A.I.; Han, S.K. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 11330–11335. [Google Scholar] [CrossRef] [PubMed]
- Boyd, K.; Shea, S.M.; Patterson, J.W. The role of capsaicin in dermatology. Prog. Drug Res. 2014, 68, 293–306. [Google Scholar] [PubMed]
- Wilson, S.R.; Bautista, D.M. Role of transient receptor potential channels in acute and chronic itch. In Itch Mechanisms and Treatment; Taylor & Francis Group, LLC.: Oxfordshire, UK, 2014. [Google Scholar]
- Polydefkis, M.; Hauer, P.; Sheth, S.; Sirdofsky, M.; Griffin, J.W.; McArthur, J.C. The time course of epidermal nerve fibre regeneration: Studies in normal controls and in people with diabetes, with and without neuropathy. Brain 2004, 127, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.S. Study on hif-1alpha gene translation in psoriatic epidermis with the topical treatment of capsaicin ointment. ISRN Pharm. 2011, 2011, 821874. [Google Scholar] [PubMed]
- Tarng, D.C.; Cho, Y.L.; Liu, H.N.; Huang, T.P. Hemodialysis-related pruritus: A double-blind, placebo-controlled, crossover study of capsaicin 0.025% cream. Nephron 1996, 72, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Weisshaar, E.; Dunker, N.; Gollnick, H. Topical capsaicin therapy in humans with hemodialysis-related pruritus. Neurosci Lett. 2003, 345, 192–194. [Google Scholar] [CrossRef]
- Sekine, R.; Satoh, T.; Takaoka, A.; Saeki, K.; Yokozeki, H. Anti pruritic effects of topical crotamiton, capsaicin, and a corticosteroid on pruritogen-induced scratching behavior. Exp. Dermatol. 2012, 21, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Lysy, J.; Sistiery-Ittah, M.; Israelit, Y.; Shmueli, A.; Strauss-Liviatan, N.; Mindrul, V.; Keret, D.; Goldin, E. Topical capsaicin—A novel and effective treatment for idiopathic intractable pruritus ani: A randomised, placebo controlled, crossover study. Gut 2003, 52, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Teofoli, P.; Tsampau, D. Treatment of aquagenic pruritus with topical capsaicin cream. J. Am. Acad. Dermatol. 1994, 30, 232–235. [Google Scholar] [CrossRef]
- Williams, S.R.; Clark, R.F.; Dunford, J.V. Contact dermatitis associated with capsaicin: Hunan hand syndrome. Ann. Emerg. Med. 1995, 25, 713–715. [Google Scholar] [CrossRef]
- Kim-Katz, S.Y.; Anderson, I.B.; Kearney, T.E.; MacDougall, C.; Hudmon, K.S.; Blanc, P.D. Topical antacid therapy for capsaicin-induced dermal pain: A poison center telephone-directed study. Am. J. Emerg. Med. 2010, 28, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Peppin, J.F.; Majors, K.; Webster, L.R.; Simpson, D.M.; Tobias, J.K.; Vanhove, G.F. Tolerability of ngx-4010, a capsaicin 8% patch for peripheral neuropathic pain. J. Pain Res. 2011, 4, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Akcay, A.B.; Ozcan, T.; Seyis, S.; Acele, A. Coronary vasospasm and acute myocardial infarction induced by a topical capsaicin patch. Turk. Kardiyol. Dern. Ars. 2009, 37, 497–500. [Google Scholar] [PubMed]
- Papoiu, A.D.; Yosipovitch, G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin. Pharmacother. 2010, 11, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Harding, L.M.; Murphy, A.; Kinnman, E.; Baranowski, A.P. Characterization of secondary hyperalgesia produced by topical capsaicin jelly—A new experimental tool for pain research. Eur. J. Pain 2001, 5, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Cho, Y.Y.; Zheng, D.; Zhu, F.; Ericson, M.E.; Ma, W.Y.; Yao, K.; Dong, Z. Transient receptor potential type vanilloid 1 suppresses skin carcinogenesis. Cancer Res. 2009, 69, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Bley, K.; Boorman, G.; Mohammad, B.; McKenzie, D.; Babbar, S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol. Pathol. 2012, 40, 847–873. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Xue, J.Y.; Jiang, A.Q.; Zhu, H.L. Capsaicin and its analogues: Structure-activity relationship study. Curr. Med. Chem. 2013, 20, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.J.; Menezes, L.M.; Silva, V.; Galhardoni, R.; Sasson, J.; Okada, M.; Duarte, K.P.; Yeng, L.T.; Andrade, D.C. Liposomal topical capsaicin in post-herpetic neuralgia: A safety pilot study. Arq. Neuropsiquiatr. 2015, 73, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Lin, Y.H.; Lu, T.M.; Wang, R.J.; Tsai, Y.H.; Wu, P.C. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int. J. Pharm. 2008, 349, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Tavano, L.; Alfano, P.; Muzzalupo, R.; de Cindio, B. Niosomes vs microemulsions: New carriers for topical delivery of capsaicin. Colloids Surf. B Biointerfaces 2011, 87, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, R.; Tavano, L.; Cassano, R.; Trombino, S.; Ferrarelli, T.; Picci, N. A new approach for the evaluation of niosomes as effective transdermal drug delivery systems. Eur. J. Pharm. Biopharm. 2011, 79, 28–35. [Google Scholar] [CrossRef] [PubMed]


| Compound * | Route | Clinical Indication | Clinical Status | References/ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| NGX-4010 (Qutenza) | Topical | PHN | FDA approved | [81] |
| PHN, HIV-DSP, and PNP | EMA approved | [24,25] | ||
| Zucapsaicin (Civamide) | Topical | PHN | Phase II | [79], NCT00845923 |
| Topical | Knee osteoarthritis | Phase III (completed) | NCT00995306, NCT00077935 | |
| Spray | Dry eye syndrome | Phase II | NCT02116244 | |
| Intranasal | Episodic cluster headache | Phase III (completed) | NCT00069082, NCT00033839 | |
| ALGRX-4975 (Adlea) | Direct instillation into the surgical site | Total knee arthroplasty | Phase III (completed) | [82], NCT00681356 |
| Injection | Postoperative pain | Phase II (completed) | NCT00133133, NCT00146198 | |
| Injection | Morton’s neuroma | Phase II (completed) | NCT00130962 | |
| RTX | Intraganglionic/intrathecal injection and topical route | Morton’s neuroma, localized nerve injuries, burns, complex regional pain syndrome, amputation, corneal neuropathic, osteoarthritic, post-incisional, lower back, and chronic gynecological pain | Phase II (completed) | [83] |
| Intrathecal injection | Cancer-induced bone pain | Phase 1b | NCT02522611 | |
| Nonivamide (Nicoboxil) | Topical | Acute lower back pain | Phase III (completed) | [84], NCT01708915 |
| Capsiate | Oral | Antitumor, anti-inflammatory, analgesic, and weight management | Phase I (completed) | [85], NCT00692601 |
| Compound * | Route | Clinical Indication | Clinical Status | References/ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| Capsazepine # | Oral | Inflammatory, neuropathic pain (Guinea pigs) | Preclinical | [86] |
| A-425619 # | Oral | Chronic inflammatory, post-operative, osteoarthritic, neuropathic pain | Preclinical | [87] |
| SB-705498 # | Oral | Acute migraine, chronic cough, dental pain | Phase II (completed) | [88], NCT00269022, NCT01476098, NCT00281684 |
| Oral | Rectal pain | Phase II (terminated) | [88], NCT00461682 | |
| Intranasal | Non-allergic- and allergic-rhinitis | Phase II (completed) | NCT01424514, NCT01424397 | |
| Topical | Atopic dermatitis | Phase I (completed) | NCT01673529 | |
| ABT-102 | Oral | Inflammatory, osteoarthritic, post-operative, and bone cancer pain | Phase I (completed) | [88,89], NCT00854659 |
| AZD1386 | Oral | Chronic pain | Phase I (completed) | NCT00736658 |
| Dental, esophageal pain | Phase II (completed) | [88], NCT01019928, NCT00672646 | ||
| Neuropathic, osteoarthritis pain | Phase II (terminated) | NCT00976534, NCT00878501 | ||
| JNJ-39439335 (Mavatrep) | Oral | Pain, osteoarthritis | Phase I (completed) | NCT01006304, NCT01343303 |
| PAC-14028 | Topical | Skin pruritus, erythematotelangiectatic and papulopustular rosacea, atopic dermatitis | Phase II (completed) | NCT02052531, NCT02052999, NCT02583022 |
| JTS-653 | Oral | Overactive bladder pain | Phase II (discontinued) | [90] |
| Post-herpetic neuralgia | Phase II (completed) | [91] | ||
| XEN-D0501 | Oral | Chronic idiopathic cough, chronic obstructive pulmonary disease (COPD) | Phase II (completed) | NCT02233699, NCT02233686 |
| Cancer Type | Tumor Cells/Cell Lines Utilized | Major Outcomes | References |
|---|---|---|---|
| Breast Cancer | MCF-7, BT-20, SKBR-3, MDA-MB231, T47D, BT-474, MCF10A | Decreased mitochondrial membrane potential, cell-cycle arrest, apoptosis | [135,136,137,138,139] |
| Cholangiocarcinoma | TFK-1 and SZ-1 | Modulation of Hedgehog pathway, apoptosis | [140] |
| Colon cancer | SW480, LoVo, HCT-116, CT-26, HT-29, CoLo320, Colo205 | Cell cycle arrest, apoptosis, changes in cell morphology, DNA fragmentation | [141,142,143,144,145] |
| Gastric cancer | AGS, SNU-668, HGC-27 | Apoptosis, inhibition of cell proliferation | [146,147,148,149] |
| Hepatocellular carcinoma | HepG2, Hep3B | Apoptosis | [150,151] |
| Pancreatic cancer | AsPC-1, BxPC-3, PANC-1 | Apoptosis | [128,152] |
| Prostate cancer | LNCaP, PC-3, DU-145 | Apoptosis, dissipation of mitochondrial inner transmembrane potential | [130,153,154] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. https://doi.org/10.3390/molecules21080966
Basith S, Cui M, Hong S, Choi S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules. 2016; 21(8):966. https://doi.org/10.3390/molecules21080966
Chicago/Turabian StyleBasith, Shaherin, Minghua Cui, Sunhye Hong, and Sun Choi. 2016. "Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases" Molecules 21, no. 8: 966. https://doi.org/10.3390/molecules21080966
APA StyleBasith, S., Cui, M., Hong, S., & Choi, S. (2016). Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules, 21(8), 966. https://doi.org/10.3390/molecules21080966