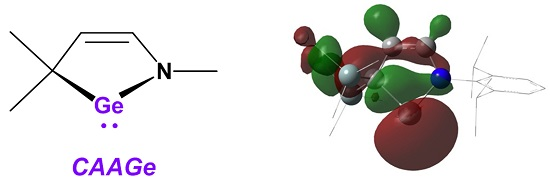Isolation of a Cyclic (Alkyl)(amino)germylene
Abstract
:1. Introduction
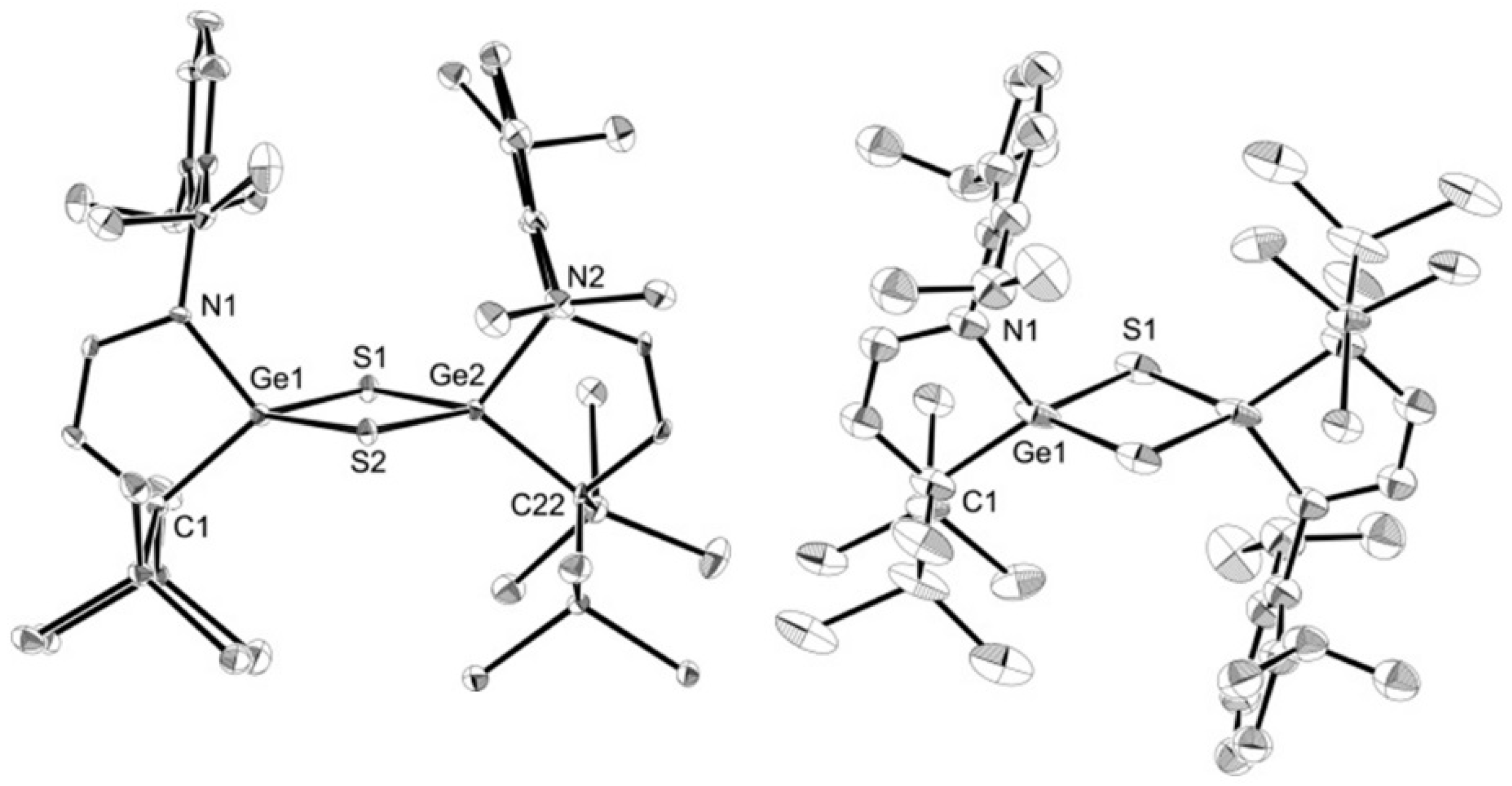
2. Results
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 1
3.3. Synthesis of 2
3.4. Synthesis of 3
3.5. Reaction of 3 with TEMPO
3.6. Reaction of 3 with N2O
3.7. Reaction of 3 with S8
3.8. X-ray Crystallography
3.9. Theoretical Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gynane, M.J.S.; Harris, D.H.; Lappert, M.F.; Power, P.P.; Riviere, P.; Riviere-Baudet, M. Subvalent group 4B metal alkyls and amides. Part 5. The synthesis and physical properties of thermally stable amides of germanium(II), tin(II), and lead(II). J. Chem. Soc. Dalton Trans. 1977, 2004–2009. [Google Scholar] [CrossRef]
- Davidson, P.J.; Harris, D.H.; Lappert, M.F. Subvalent group 4B metal alkyls and amides. Part I. The synthesis and physical properties of kinetically stable bis[bis(trimethysilyl)methyl]-germanium(II), -tin(II), and -lead(II). J. Chem. Soc. Dalton Trans. 1976, 2268–2274. [Google Scholar] [CrossRef]
- Harris, D.H.; Lappert, M.F. Monomeric, volatile bivalent amides of group IVB elements, M(NR12)2 and M(NR1R2)2 (M = Ge, Sn, or Pb; R1 = Me3Si, R2 = Me3C). J. Chem. Soc. Chem. Commun. 1974, 895–896. [Google Scholar] [CrossRef]
- Asay, M.; Jones, C.; Driess, M. N-Heterocyclic carbene analogues with low-valent group 13 and group 14 elements: Syntheses, structures, and reactivities of a new generation of multitalented ligands. Chem. Rev. 2011, 111, 354–396. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.C.; Power, P.P. π-Bonding and the lone pair effect in multiple bonds involving heavier main group elements: Developments in the new millennium. Chem. Rev. 2010, 110, 3877–3923. [Google Scholar] [CrossRef] [PubMed]
- Mizuhata, Y.; Sasamori, T.; Tokitoh, N. Stable heavier carbene analogues. Chem. Rev. 2009, 109, 3479–3511. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, S.; Roesky, H.W. The chemistry of aluminum(I), silicon(II), and germanium(II). Organometallics 2008, 27, 457–492. [Google Scholar] [CrossRef]
- Zabula, A.V.; Hahn, F.E. Mono- and bidentate benzannulated N-heterocyclic germylenes, stannylenes and plumbylenes. Eur. J. Inorg. Chem. 2008, 2008, 5165–5179. [Google Scholar] [CrossRef]
- Lappert, M.; Protchenko, A.; Power, P.; Seeber, A. Subvalent amides of silicon and the group 14 metals. In Metal Amide Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 263–326. [Google Scholar]
- Herrmann, W.A.; Denk, M.; Behm, J.; Scherer, W.; Klingan, F.-R.; Bock, H.; Solouki, B.; Wagner, M. Stable cyclic germanediyls (“cyclogermylenes”): Synthesis, structure, metal complexes, and thermolyses. Angew. Chem. Int. Ed. Engl. 1992, 31, 1485–1488. [Google Scholar] [CrossRef]
- West, R.; Moser, D.F.; Guzei, I.A.; Lee, G.-H.; Naka, A.; Li, W.; Zabula, A.; Bukalov, S.; Leites, L. The surprising reactions of 1,3-di-tert-butyl-2,2-dichloro-1,3-diaza-2-germa-4-cyclopentene. Organometallics 2006, 25, 2709–2711. [Google Scholar] [CrossRef]
- Gallego, D.; Brück, A.; Irran, E.; Meier, F.; Kaupp, M.; Driess, M.; Hartwig, J.F. From bis(silylene) and bis(germylene) pincer-type nickel(II) complexes to isolable intermediates of the nickel-catalyzed sonogashira cross-coupling reaction. J. Am. Chem. Soc. 2013, 135, 15617–15626. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Objartel, I.; Roesky, H.W.; Stalke, D. Cleavage of a N−H bond of ammonia at room temperature by a germylene. Inorg. Chem. 2009, 48, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Iwata, S.; Hiraishi, M. Novel 2:1 periodic copolymers from cyclic germylenes and p-benzoquinone derivatives. J. Am. Chem. Soc. 1994, 116, 6047–6048. [Google Scholar] [CrossRef]
- Shoda, S.-I.; Iwata, S.; Kim, H.J.; Hiraishi, M.; Kobayashi, S. Poly(germanium thiolate): A new class of organometallic polymers having a germanium-sulfur bond in the main chain. Macromol. Chem. Phys. 1996, 197, 2437–2445. [Google Scholar] [CrossRef]
- Shoda, S.-i.; Iwata, S.; Yajima, K.; Yagi, K.; Ohnishi, Y.; Kobayashi, S. Synthesis of germanium enolate polymers from germylene monomers. Tetrahedron 1997, 53, 15281–15295. [Google Scholar] [CrossRef]
- Al-Rafia, S.M.I.; Malcolm, A.C.; Liew, S.K.; Ferguson, M.J.; Rivard, E. Stabilization of the heavy methylene analogues, GeH2 and SnH2, within the coordination sphere of a transition metal. J. Am. Chem. Soc. 2011, 133, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Tsubota, T.; Fukuda, T.; Tobita, H. Synthesis and structure of a hydrido(hydrogermylene)tungsten complex and its reactions with nitriles and ketones. Chem. Lett. 2009, 38, 1196–1197. [Google Scholar] [CrossRef]
- Hlina, J.; Baumgartner, J.; Marschner, C.; Zark, P.; Müller, T. Coordination chemistry of disilylated germylenes with group 4 metallocenes. Organometallics 2013, 32, 3300–3308. [Google Scholar] [CrossRef] [PubMed]
- Brück, A.; Gallego, D.; Wang, W.; Irran, E.; Driess, M.; Hartwig, J.F. Pushing the σ-donor strength in iridium pincer complexes: Bis(silylene) and bis(germylene) ligands are stronger donors than bis(phosphorus(III)) ligands. Angew. Chem. Int. Ed. 2012, 51, 11478–11482. [Google Scholar] [CrossRef] [PubMed]
- Lappert, M.F.; Rowe, R.S. The role of group 14 element carbene analogues in transition metal chemistry. Coord. Chem. Rev. 1990, 100, 267–292. [Google Scholar] [CrossRef]
- Petz, W. Transition-Metal complexes with derivatives of divalent silicon, germanium, tin, and lead as ligands. Chem. Rev. 1986, 86, 1019–1047. [Google Scholar] [CrossRef]
- Li, Y.; Mondal, K.C.; Stollberg, P.; Zhu, H.; Roesky, H.W.; Herbst-Irmer, R.; Stalke, D.; Fliegl, H. Unusual formation of a N-heterocyclic germylene via homolytic cleavage of a C-C bond. Chem. Commun. 2014, 50, 3356–3358. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.J.; Jones, C.; Mills, D.P.; Pierce, G.A.; Waugh, M. Investigations into the preparation of groups 13–15 N-heterocyclic carbene analogues. Inorg. Chim. Acta 2008, 361, 427–435. [Google Scholar] [CrossRef]
- Heinicke, J.; Oprea, A.; Kindermann, M.K.; Karpati, T.; Nyulászi, L.; Veszprémi, T. Unsymmetrical carbene homologues: Isolable pyrido[b]-1,3,2λ2-diazasilole, -germole and -stannole and quantum-chemical comparison with unstable pyrido[c] isomers. Chem. Eur. J. 1998, 4, 541–545. [Google Scholar] [CrossRef]
- Tomasik, A.C.; Hill, N.J.; West, R. Synthesis and characterization of three new thermally stable N-heterocyclic germylenes. J. Organomet. Chem. 2009, 694, 2122–2125. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Skatova, A.A.; Chudakova, V.A.; Khvoinova, N.M.; Baurin, A.Y.; Dechert, S.; Hummert, M.; Schumann, H. Stable germylenes derived from 1,2-bis(arylimino)acenaphthenes. Organometallics 2004, 23, 3714–3718. [Google Scholar] [CrossRef]
- Ullah, F.; Oprea, A.I.; Kindermann, M.K.; Bajor, G.; Veszprémi, T.; Heinicke, J. Homologues of N-heterocyclic carbenes: Detection and electronic structure of N-bridgehead pyrido[a]-anellated 1,3,2-diazagermol-2-ylidenes. J. Organomet. Chem. 2009, 694, 397–403. [Google Scholar] [CrossRef]
- Huang, M.; Kireenko, M.M.; Zaitsev, K.V.; Oprunenko, Y.F.; Churakov, A.V.; Howard, J.A.K.; Lermontova, E.K.; Sorokin, D.; Linder, T.; Sundermeyer, J.; et al. Stabilized germylenes based on diethylenetriamines and related diamines: Synthesis, structures, and chemical properties. Eur. J. Inorg. Chem. 2012, 2012, 3712–3724. [Google Scholar] [CrossRef]
- Karwasara, S.; Yadav, D.; Jha, C.K.; Rajaraman, G.; Nagendran, S. Single-Step conversion of silathiogermylene to germaacid anhydrides: Unusual reactivity. Chem. Commun. 2015, 51, 4310–4313. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Tokitoh, N. Heavy ketones, the heavier element congeners of a ketone. Acc. Chem. Res. 2000, 33, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.-P.; Chiu, W.-K.; Chong, K.-H.; Mak, T.C.W. Synthesis of a germanium analogue of a dithiocarboxylic acid anhydride from the Ge(I) pyridyl-1-azaallyl dimer. Chem. Commun. 2009, 6822–6824. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ma, Q.; Usón, I.; Roesky, H.W.; Noltemeyer, M.; Schmidt, H.-G. Synthesis and structures of [{HC(CMeNAr)2}Ge(S)X] (Ar = 2,6-iPr2C6H3, X = F, Cl, Me): Structurally characterized examples with a formal double bond between group 14 and 16 elements bearing a halide. J. Am. Chem. Soc. 2002, 124, 8542–8543. [Google Scholar] [CrossRef] [PubMed]
- Kira, M.; Ishida, S.; Iwamoto, T.; Ichinohe, M.; Kabuto, C.; Ignatovich, L.; Sakurai, H. Synthesis and structure of a stable cyclic dialkylgermylene. Chem. Lett. 1999, 28, 263–264. [Google Scholar] [CrossRef]
- Martin, D.; Soleilhavoup, M.; Bertrand, G. Stable singlet carbenes as mimics for transition metal centers. Chem. Sci. 2011, 2, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.D.; Soleilhavoup, M.; Bertrand, G. Carbene-Stabilized main group radicals and radical ions. Chem. Sci. 2013, 4, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Soleilhavoup, M.; Bertrand, G. Cyclic (alkyl)(amino)carbenes (CAACs): Stable carbenes on the rise. Acc. Chem. Res. 2015, 48, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Melaimi, M.; Soleilhavoup, M.; Bertrand, G. Stable cyclic carbenes and related species beyond diaminocarbenes. Angew. Chem. Int. Ed. 2010, 49, 8810–8849. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Melaimi, M.; Soleilhavoup, M.; Bertrand, G. A brief survey of our contribution to stable carbene chemistry. Organometallics 2011, 30, 5304–5313. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Mondal, K.C.; Roesky, H.W. Cyclic alkyl(amino) carbene stabilized complexes with low coordinate metals of enduring nature. Acc. Chem. Res. 2016, 49, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Al-Rafia, S.M.I.; Momeni, M.R.; McDonald, R.; Ferguson, M.J.; Brown, A.; Rivard, E. Controlled growth of dichlorogermanium oligomers from lewis basic hosts. Angew. Chem. Int. Ed. 2013, 52, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Tokitoh, N.; Manmaru, K.; Okazaki, R. Synthesis and crystal structure of the first base-free diarylgermylene-transition metal mononuclear complexes. Organometallics 1994, 13, 167–171. [Google Scholar] [CrossRef]
- Naka, A.; Hill, N.J.; West, R. Free radical reactions of stable silylenes and germylenes. Organometallics 2004, 23, 6330–6332. [Google Scholar] [CrossRef]
- Tokitoh, N.; Matsumoto, T.; Okazaki, R. Formation and reactions of the first diarylgermanone stable in solution. Chem. Lett. 1995, 24, 1087–1088. [Google Scholar] [CrossRef]
- Jutzi, P.; Schmidt, H.; Neumann, B.; Stammler, H.-G. Bis(2,4,6-tri-tert-butylphenyl)germylene reinvestigated: Crystal structure, lewis acid catalyzed C−H insertion, and oxidation to an unstable germanone. Organometallics 1996, 15, 741–746. [Google Scholar] [CrossRef]
- Iwamoto, T.; Masuda, H.; Ishida, S.; Kabuto, C.; Kira, M. Diverse reactions of nitroxide-radical adducts of silylene, germylene, and stannylene. J. Organomet. Chem. 2004, 689, 1337–1341. [Google Scholar] [CrossRef]
- Iwamoto, T.; Masuda, H.; Ishida, S.; Kabuto, C.; Kira, M. Addition of stable nitroxide radical to stable divalent compounds of heavier group 14 elements. J. Am. Chem. Soc. 2003, 125, 9300–9301. [Google Scholar] [CrossRef] [PubMed]
- Spikes, G.H.; Peng, Y.; Fettinger, J.C.; Steiner, J.; Power, P.P. Different reactivity of the heavier group 14 element alkyne analogues Ar′MMAr′ (M = Ge, Sn; Ar′ = C6H3–2,6(C6H3–2,6-Pri2)2) with R2NO. Chem. Commun. 2005, 6041–6043. [Google Scholar] [CrossRef] [PubMed]
- Ramaker, G.; Schäfer, A.; Saak, W.; Weidenbruch, M. One-Pot synthesis of a tetragermabutadiene and its reactions with oxygen and sulfur. Organometallics 2003, 22, 1302–1304. [Google Scholar] [CrossRef]
- Ellis, D.; Hitchcock, P.B.; Lappert, M.F. Preparation and X-ray structure of [Ge{N(SiMe3)2}2(µ-O)]2, a rare 1,3-cyclodigermoxane. J. Chem. Soc. Dalton Trans. 1992, 3397–3398. [Google Scholar] [CrossRef]
- Masamune, S.; Batcheller, S.A.; Park, J.; Davis, W.M.; Yamashita, O.; Ohta, Y.; Kabe, Y. Oxygenation of digermene derivatives. J. Am. Chem. Soc. 1989, 111, 1888–1889. [Google Scholar] [CrossRef]
- Jana, A.; Roesky, H.W.; Schulzke, C. Reactivity of germanium(II) hydride with nitrous oxide, trimethylsilyl azide, ketones, and alkynes and the reaction of a methyl analogue with trimethylsilyl diazomethane. Dalton Trans. 2010, 39, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Xiong, Y.; Driess, M. From NHC→germylenes to stable NHC→germanone complexes. Chem. Commun. 2009, 6466–6468. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Xiong, Y.; Wang, W.; Driess, M. Synthesis, structure, and reactivity of a pyridine-stabilized germanone. Chem. Eur. J. 2011, 17, 4890–4895. [Google Scholar] [CrossRef] [PubMed]
- Driess, M.; Yao, S.; Brym, M.; van Wüllen, C. A heterofulvene-like germylene with a betain reactivity. Angew. Chem. Int. Ed. 2006, 45, 4349–4352. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Inoue, S.; Enthaler, S.; Driess, M. Bis(silylenyl)- and bis(germylenyl)-substituted ferrocenes: Synthesis, structure, and catalytic applications of bidentate silicon(II)–cobalt complexes. Angew. Chem. Int. Ed. 2012, 51, 6167–6171. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fukawa, T.; Matsuo, T.; Hashizume, D.; Fueno, H.; Tanaka, K.; Tamao, K. A stable germanone as the first isolated heavy ketone with a terminal oxygen atom. Nat. Chem. 2012, 4, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.K.; Al-Rafia, S.M.I.; Goettel, J.T.; Lummis, P.A.; McDonald, S.M.; Miedema, L.J.; Ferguson, M.J.; McDonald, R.; Rivard, E. Expanding the steric coverage offered by bis(amidosilyl) chelates: Isolation of low-coordinate n-heterocyclic germylene complexes. Inorg. Chem. 2012, 51, 5471–5480. [Google Scholar] [CrossRef] [PubMed]
- Al-Rafia, S.M.I.; Lummis, P.A.; Ferguson, M.J.; McDonald, R.; Rivard, E. Low-Coordinate germylene and stannylene heterocycles featuring sterically tunable bis(amido)silyl ligands. Inorg. Chem. 2010, 49, 9709–9717. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, P.; Yap, G.P.A.; Richeson, D.S. Synthesis and properties of a germanium(II) metalloheterocycle derived from 1,8-di(isopropylamino)naphthalene. A novel ligand leading to formation of Ni{Ge[(iPrN)2C10H6]}4. J. Am. Chem. Soc. 2001, 123, 11162–11167. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tokitoh, N.; Okazaki, R. The first kinetically stabilized germanethiones and germaneselones: Syntheses, structures, and reactivities. J. Am. Chem. Soc. 1999, 121, 8811–8824. [Google Scholar] [CrossRef]
- Tokitoh, N.; Matsumoto, T.; Manmaru, K.; Okazaki, R. Synthesis and crystal structure of the first stable diarylgermanethione. J. Am. Chem. Soc. 1993, 115, 8855–8856. [Google Scholar] [CrossRef]
- Xiong, Y.; Yao, S.; Karni, M.; Kostenko, A.; Burchert, A.; Apeloig, Y.; Driess, M. Heavier congeners of CO and CO2 as ligands: From zero-valent germanium (‘germylone’) to isolable monomeric GeX and GeX2 complexes (X = S, Se, Te). Chem. Sci. 2016, 7, 5462–5469. [Google Scholar] [CrossRef]
- Sinhababu, S.; Yadav, D.; Karwasara, S.; Sharma, M.K.; Mukherjee, G.; Rajaraman, G.; Nagendran, S. The preparation of complexes of germanone from a germanium μ-oxo dimer. Angew. Chem. Int. Ed. 2016, 55, 7742–7746. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yao, S.; Driess, M. Chemical tricks to stabilize silanones and their heavier homologues with E=O bonds (E = Si–Pb): From elusive species to isolable building blocks. Angew. Chem. Int. Ed. 2013, 52, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Sun, X.; Li, H.; Song, Z.; Liu, Z. Geminal bis(silyl) enal: A versatile scaffold for stereoselective synthesizing C3,O1-disilylated allylic alcohols based upon anion relay chemistry. Org. Lett. 2013, 15, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- SHELX-2013. Available online: http://shelx.uni-ac.gwdg.de/SHELX/index.php (accessed on 15 July 2014).
- Gaussian 09; Revision B.01; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. (Eds.) Gaussian, Inc.: Wallingford, CT, USA, 2010.
- Sample Availability: Samples of the compounds are not available from the authors.








© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Lim, Y.S.; Li, Y.; Ganguly, R.; Kinjo, R. Isolation of a Cyclic (Alkyl)(amino)germylene. Molecules 2016, 21, 990. https://doi.org/10.3390/molecules21080990
Wang L, Lim YS, Li Y, Ganguly R, Kinjo R. Isolation of a Cyclic (Alkyl)(amino)germylene. Molecules. 2016; 21(8):990. https://doi.org/10.3390/molecules21080990
Chicago/Turabian StyleWang, Liliang, Yi Shan Lim, Yongxin Li, Rakesh Ganguly, and Rei Kinjo. 2016. "Isolation of a Cyclic (Alkyl)(amino)germylene" Molecules 21, no. 8: 990. https://doi.org/10.3390/molecules21080990
APA StyleWang, L., Lim, Y. S., Li, Y., Ganguly, R., & Kinjo, R. (2016). Isolation of a Cyclic (Alkyl)(amino)germylene. Molecules, 21(8), 990. https://doi.org/10.3390/molecules21080990