Abstract
Three new triterpenoid alkaloids, namely buxmicrophyllines P–R (1–3), were isolated from the twigs and leaves of Buxus microphylla. Their structures were elucidated on the basis of NMR and MS spectroscopic analyses. Structurally, compounds 1–3 belong to the 9,10-cycloartane type alkaloids. In addition, compound 3 exhibited moderate cytotoxic activities in vitro against HL-60, SMMC-7221, A-549, MCF-7, and SW480 cell lines (with IC50 values ranging from 4.51 to 15.58 μM).
1. Introduction
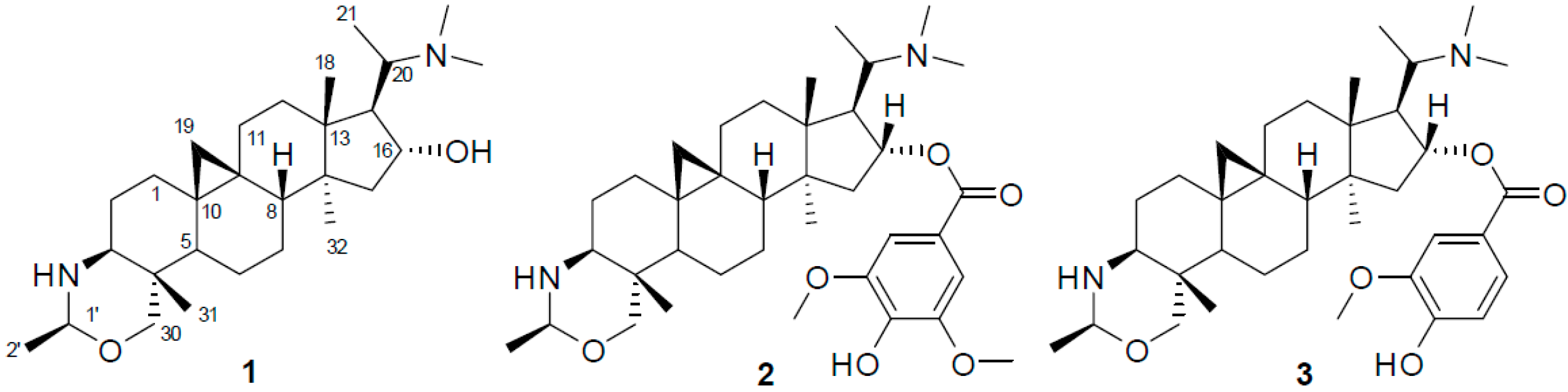
Plants of the genus Buxus are abundant in triterpenoid alkaloids (Buxus alkaloids), comprised of more than 140 analogues with a 9,10-cyclopropyl ring system and a degraded C-20 side chain [1]. Some of these alkaloids have been demonstrated to have antimalarial, antituberculosis, anti-HIV, and anticancer activities [2,3,4,5,6,7,8,9]. One of these plants, B. microphylla Sieb. et Zucc. (Buxaceae), native to Southern China, is an evergreen shrub and usually planted to beautify the environment [10]. Moreover, twigs and leaves of this plant are used in folkloric medicine for the treatment of tumor, stomachache, hernia, and acute myocardial ischemia [10]. In our continuous search for active alkaloids from this plant [11,12,13], three new triterpenoid alkaloids, namely buxmicrophyllines P–R (1–3), were isolated from the twigs and leaves of B. microphylla. The three compounds (shown in Figure 1) were evaluated for their cytotoxic activities in five human tumor cell lines. Herein, we described the isolation, structure elucidation, and cytotoxicity of these compounds.
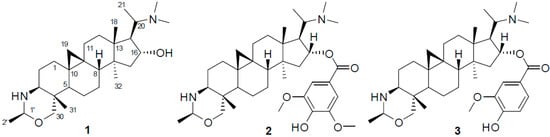
Figure 1.
Chemical structures of compounds 1–3.
2. Results and Discussion
Three new triterpenoid alkaloids (1–3) were obtained by chromatographic separation of the acetone extract of the twigs and leaves of B. microphylla.
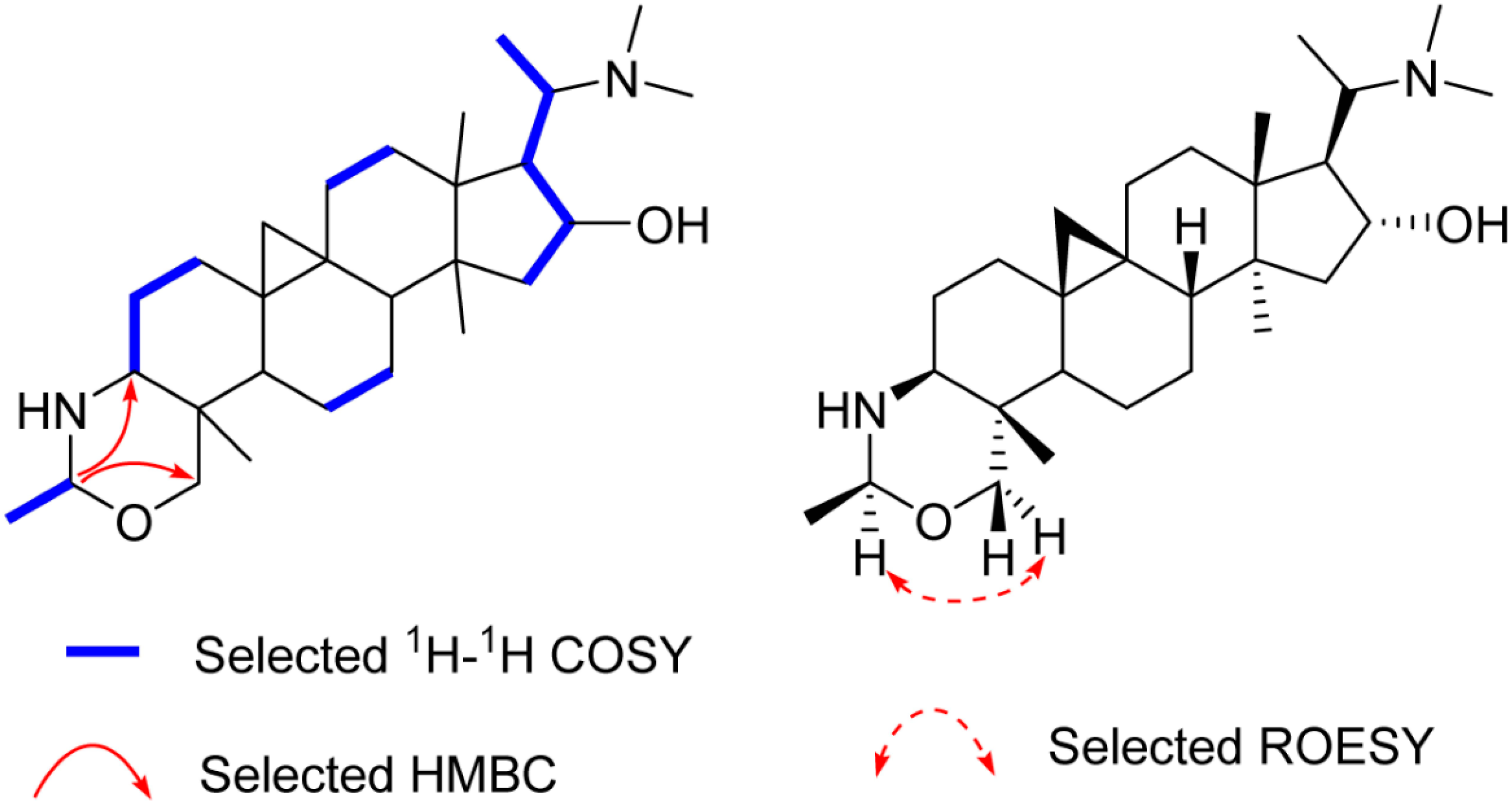
Compound 1, an amorphous powder, gave a molecular formula C28H48N2O on the basis of High Resolution Electrospray Ionization Mass Spectroscopy (HR-ESI-MS) spectrum (m/z 445.3795 [M + H]+, calculated for C28H49N2O, 445.3797). The 1H- and 13C-DEPT NMR spectra (Table 1 and Supplementary Materials Figures S1–S6) of 1 displayed signals for seven methyls (three tertiary singlets at δH 0.96, 0.98, and 1.12), nine methylenes (two typical cyclopropyl protons at δH 0.59 (d, J = 4.0 Hz) and 0.33 (d, J = 4.0 Hz)), seven methines, and five quaternary carbons. These functionalities suggested that 1 is a typical 9β,10β-cycloartane type triterpenoid alkaloid [12]. Further analysis of the NMR data revealed that the structure of 1 was parallel to that of cyclobuxoxazine [12,14], except for the presence of an additional secondary methyl group (δC 21.7 and δH 1.30 (d, J = 5.5 Hz)) and a methine group (δC 85.3 and δH 4.29) replacing the methylene group (δC 79.5) at C-1′ in the latter, suggesting that the methyl group should be located at C-1′. This deduction could be further confirmed by the 1H-Detected Heteronuclear Multiple Bond Correlation (HMBC) of H-1′ to C-3 and C-30 and the 1H-1H Correlation Spectroscopy (1H-1H COSY) of H-2′/H-1′ (Figure 2). The H-1′ proton was assigned as α-oriented by the Rotating-frame Overhauser Enhancement Spectroscopy (ROESY) from H-1′ to Hα-30 (Figure 2). Thereby, the structure of 1 was defined as shown and named buxmicrophylline P.

Table 1.
1H-NMR and 13C-NMR data of compounds 1–3 a.
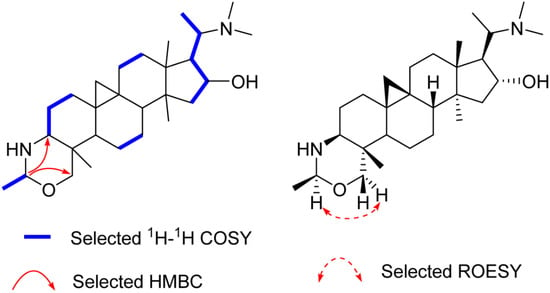
Figure 2.
Key 2D correlations of 1.
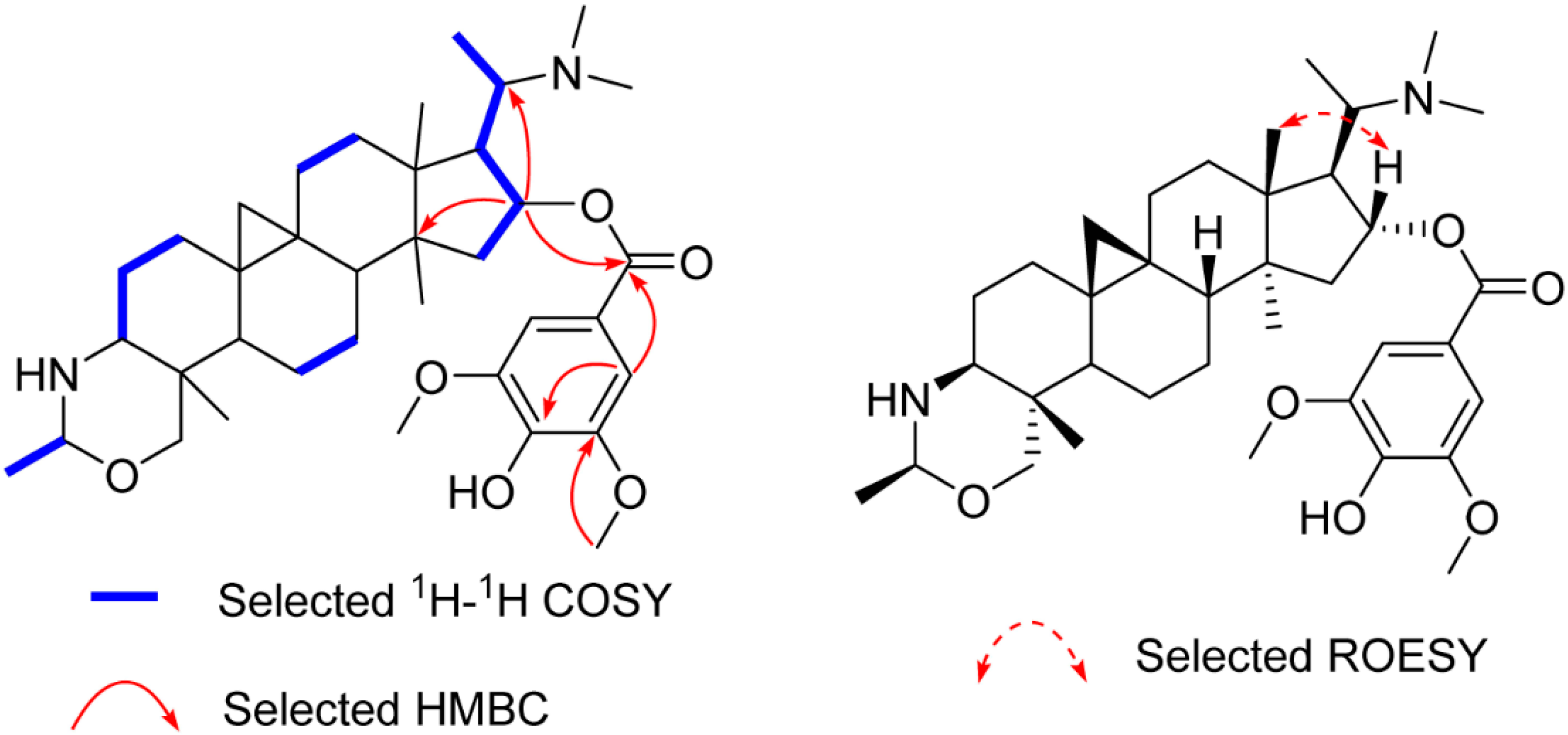
Compound 2 yielded the molecular formula C37H56N2O6 based on its 13C-NMR and the HR-ESI-MS ion peak at m/z 625.4216 [M + H]+ (calculated 625.4212), 180 mass units more than that of 1, suggesting that it is a syringoylated derivative of 1. The additional syringoyl group (δH 7.27 (s, 2H); δC 165.8, 121.8, 106.5, 146.7, 139.1) and the downfiled shift of C-16 (from δC 78.9 to δC 80.4) allowed the location of the syringoyl group at C-16, as confirmed by the HMBC correlations of H-16 (δH 5.26) to the carbonyl carbon (δC 165.8) of the syringoyl group, C-20, and C-14 (Figure 3). The β-orientation of H-16 was assigned as that of 1 by ROESY correlations of H-16/H-18 (Figure 3). The structure of 2 (buxmicrophylline Q) was therefore depicted as shown.
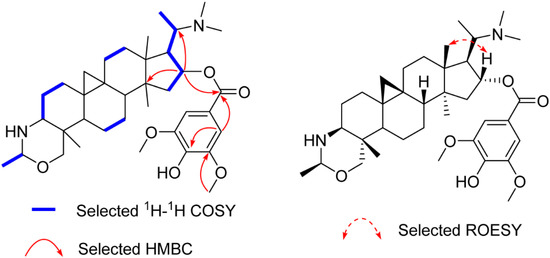
Figure 3.
Key 2D correlations of 2.
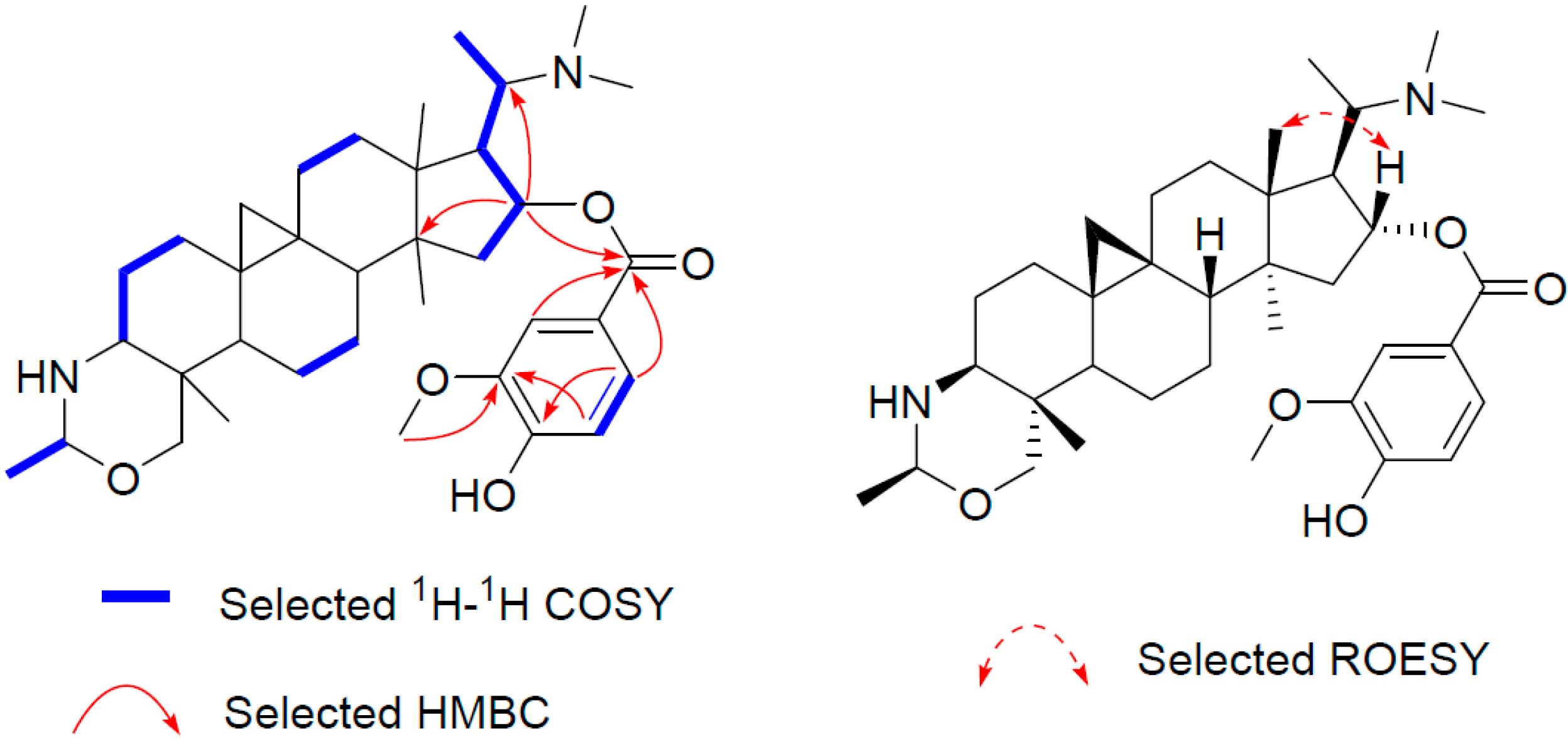
Similarly, the structure of compound 3 (buxmicrophylline R), which has the molecular formula C36H54N2O5 as determined by the HR-ESI-MS ion peak at 595.4111 [M + H]+ (calculated 595.4106), was established by comparing its NMR data with those of 1 and 2. It turned out that there was a vanilloyl group (δH 7.60, 7.57, 6.91; δC 165.8, 123.4, 111.8, 149.5, 146.1, 113.8, 123.7) in 3 rather than a syringoyl group. The location of the vanilloyl group was also at C-16, as confirmed from the HMBC cross peaks of H-16 with the carbonyl carbon (δC 165.8, Figure 4).
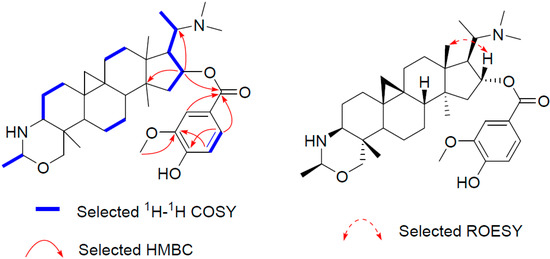
Figure 4.
Key 2D correlations of 3.
Compounds 1–3 were tested for their cytotoxic effects against five human tumor cell lines (Table 2). Compared with the positive control cisplatin, compound 3 displayed the most potent cytotoxicity against MCF-7 cells with IC50 values of 4.51 μM. However, the other tested compounds did not exert any cytotoxic effect, even at 40 μM.

Table 2.
Cytotoxic activities of compounds 1–3 with IC50 values (μM).
3. Experimental Section
3.1. General Information
Optical rotations were measured with a JASCO P-1020 polarimeter (JASCO Corporation, Tokyo, Japan). UV spectra were obtained using a Shimadzu UV 2401PC instrument (Shimadzu, Tokyo, Japan). Infrared spectra were recorded on a Bruker Tensor-27 instrument (Bruker, Zurich, Switzerland) by using KBr pellets. 1D and 2D-NMR experiments were performed on Bruker AV-400 and DRX-500 instruments (Bruker) with TMS as internal standard. HR-ESI-MS data were acquired on an API QSTAR Pulsar spectrometer (Applied Biosystems, Carlsbad, CA, USA). Column chromatography (CC) was performed on SiO2 (200–300 mesh, Qingdao Marine Chemical Group Corporation, Qingdao, China).
3.2. Plant Material
The twigs and leaves of B. microphylla were collected from Kunming, Yunnan Province, China, in September 2013, and identified by Zong-Yu Wang (Kunming Institute of Botany, Yunnan, China). A voucher specimen (KIB. Bm-20130915) has been deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
3.3. Extraction and Isolation
The chopped, dried plant material of B. microphylla (10.0 kg) was extracted three times with acetone (20 L) at room temperature, seven days each time. The filtrate was concentrated under reduced pressure to yield a residue (500 g), which was further suspended in 0.001 N HCl and partitioned with ethyl acetate (EtOAc). The aqueous layer was alkalinized to pH 10.0 with 2 N NaOH followed by exhaustive extraction with CHCl3. The CHCl3-soluble fraction (120 g) was chromatographed on a silica gel column, eluted with CHCl3–MeOH (100:0, 50:1, 20:1, 10:1, 2:1) to give five fractions, A1–A5. Fraction A4 (12 g) was subjected to further silica gel column chromatography using petroleum ether–EtOAc–diethylamine (20:1:1, 10:1:1, 5:1:1, 2:1:1), to yield 1 (20 mg), 2 (4 mg), 3 (5 mg).
Buxmicrophylline P (1): White amorphous powder; +6.8 (c = 0.18, MeOH); UV (MeOH) λmax (log ε) 240 (2.13) nm; IR (KBr) νmax 3426, 2938, 2870, 2719, 1634, 1460cm−1; 1H and 13C-NMR data, see Table 1; HRESIMS m/z 445.3795 (calcd. for C28H49N2O, 445.3797).
Buxmicrophylline Q (2): White amorphous powder; −1.8 (c = 0.29, MeOH); UV (MeOH) λmax (log ε) 270 (2.49) nm; IR (KBr) νmax 3429, 2936, 2866, 1708, 1460cm−1; 1H and 13C-NMR data, see Table 1; HRESIMS m/z 625.4216 (calcd. for C37H57N2O6, 625.4212).
Buxmicrophylline R (3): White amorphous powder; −13.7 (c = 0.15, MeOH); UV (MeOH) λmax (log ε) 240 (2.56), 265 (2.44) nm; IR (KBr) νmax 3430, 2936, 1710, 1632, 1461, 1292 cm−1; 1H and 13C-NMR data, see Table 1; HRESIMS m/z 595.4111 (calcd. for C36H55N2O5, 595.4106).
3.4. Cytotoxicity Assay
Compounds 1–3 were tested in vitro for their cytotoxicities against five human tumor cell lines (promyelocytic leukemia HL-60, hepatocellular carcinoma SMMC-7721, lung adenocarcinoma A-549, breast cancer MCF-7, and colon adenocarcinoma SW480) by the microculture tetrazolium (MTT) assay. Cytotoxicity evaluations were performed based on the previously described protocol [12], with cisplatin as the positive control. Briefly, after 24 h incubation in 96-well plates, each tumor cell line was exposed to 1–3 or positive control at final concentrations of 1, 2, 5, 10, 20, and 40 μM for 72 h. At the end of exposure, 20 μL of 5 mg/mL MTT (Sigma-Aldrich, St. Louis, MO, USA) was added to each well, and the plates were incubated for another 4 h. Then, 100 μL 20% SDS was added, and the plates were further incubated for 12 h. The optical density (OD) was read on a plate reader at 570 nm. IC50 values were expressed as concentration of a compound reducing cell growth by 50%. All samples were assayed in triplicate.
4. Conclusions
Phytochemical investigations of B. microphylla afforded three new 9,19-cycloartane type alkaloids, buxmicrophyllines P–R (1–3), together with a known analogue, buxbodine B. In vitro cytotoxicity assay proved that compound 3 exhibited more potent cytotoxic activity against MCF-7 cell line than the positive control, making this compound a potential lead entity for further study.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/9/1125/s1.
Acknowledgments
The project was financially supported by the Youth Program of National Natural Science Foundation of China (NSFC, No. 81403050 to L.S.W.), and Foundation of State of Key Laboratory of Phytochemistry and Plant Resources in West China (P2015-ZZ09).
Author Contributions
Ming-Hua Qiu conceived and designed the experiments; Shi-Tou Bai, Guo-Lei Zhu, Xing-Rong Peng, Jin-Run Dong, Mu-Yuan Yu and Jian-Chao Chen performed the experiments; Luo-Sheng Wan analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, J.; Qin, X.Y.; Zhang, S.D.; Xu, X.S.; Pei, J.P.; Fu, J.J. Chemical constituents of plants from the Genus Buxus. Chem. Biodivers. 2015, 12, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.X.; Sun, Y.; Chen, J.C.; Qiu, M.H. Triterpenoid alkaloid derivatives from Buxus rugulosa. Nat. Prod. Bioprospect. 2011, 1, 71–72. [Google Scholar] [CrossRef]
- Yan, Y.X.; Sun, Y.; Chen, J.C.; Qiu, M.H. A new triterpenoid alkaloid from Buxus sempervirens. Z. Naturforsch. B 2011, 66, 1076–1078. [Google Scholar] [CrossRef]
- Yan, Y.X.; Sun, Y.; Li, Z.R.; Zhou, L.; Qiu, M.H. Chemistry and biological activities of Buxus Alkaloids. Curr. Bioact. Compd. 2011, 7, 47–64. [Google Scholar] [CrossRef]
- Lam, C.W.; Wakeman, A.; James, A.; Ata, A. Bioactive steroidal alkaloids from Buxus macowanii Oliv. Steroids 2015, 95, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.U.; Naz, S.; Ata, A.; Choudhary, M.I.; Sener, B.; Turkoz, S. Novel triterpenoidal alkaloids from the roots of Buxus sempervirens. Nat. Prod. Lett. 1998, 12, 299–306. [Google Scholar] [CrossRef]
- Atta-ur-Rahmna; Ata, A.; Naz, S.; Choudhary, M.I.; Sener, B.; Turkoz, S. New steroidal alkaloids from the roots of Buxus sempervirens. J. Nat. Prod. 1999, 62, 665–669. [Google Scholar]
- Qiu, M.H.; Yang, W.S.; Nie, R.L. Steroidal alkaloids from Buxus bodinieri. Acta Bot. Sin. 2001, 23, 357–362. [Google Scholar]
- Ata, A.; Naz, S.; Choudhary, M.I.; Atta-ur-Rahman; Sener, B.; Turkoz, S. New triterpenoidal alkaloids from Buxus sempervirens. Z. Naturforsch. C 2002, 57, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chiu, M.H.; Nie, R.L. Two new buxus alkaloids from Buxus microphylla. Acta Bot. Sin. 1996, 38, 483–488. [Google Scholar]
- Du, J.; Chiu, M.H.; Nie, R.L. Three steroidal alkaloids from Buxus microphylla. J. Asian Nat. Prod. Res. 1999, 1, 239–244. [Google Scholar] [CrossRef]
- Yan, Y.X.; Sun, Y.; Chen, J.C.; Qiu, M.H. Cytotoxic triterpenoid alkaloids from Buxus microphylla. J. Nat. Prod. 2009, 72, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.X.; Chen, J.C; Sun, Y.; Wang, Y.Y.; Su, J.; Li, Y.; Qiu, M.H. Triterpenoid alkaloids from Buxus microphylla. Chem. Biodivers. 2010, 7, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Terao, S. Buxus alkaloids. V. Constitution of cyclobuxoxazine, a new skeletal alkaloid containing a tetrahydrooxazine ring. J. Chem. Soc. 1965, 8, 4537–4542. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1 and 3 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).