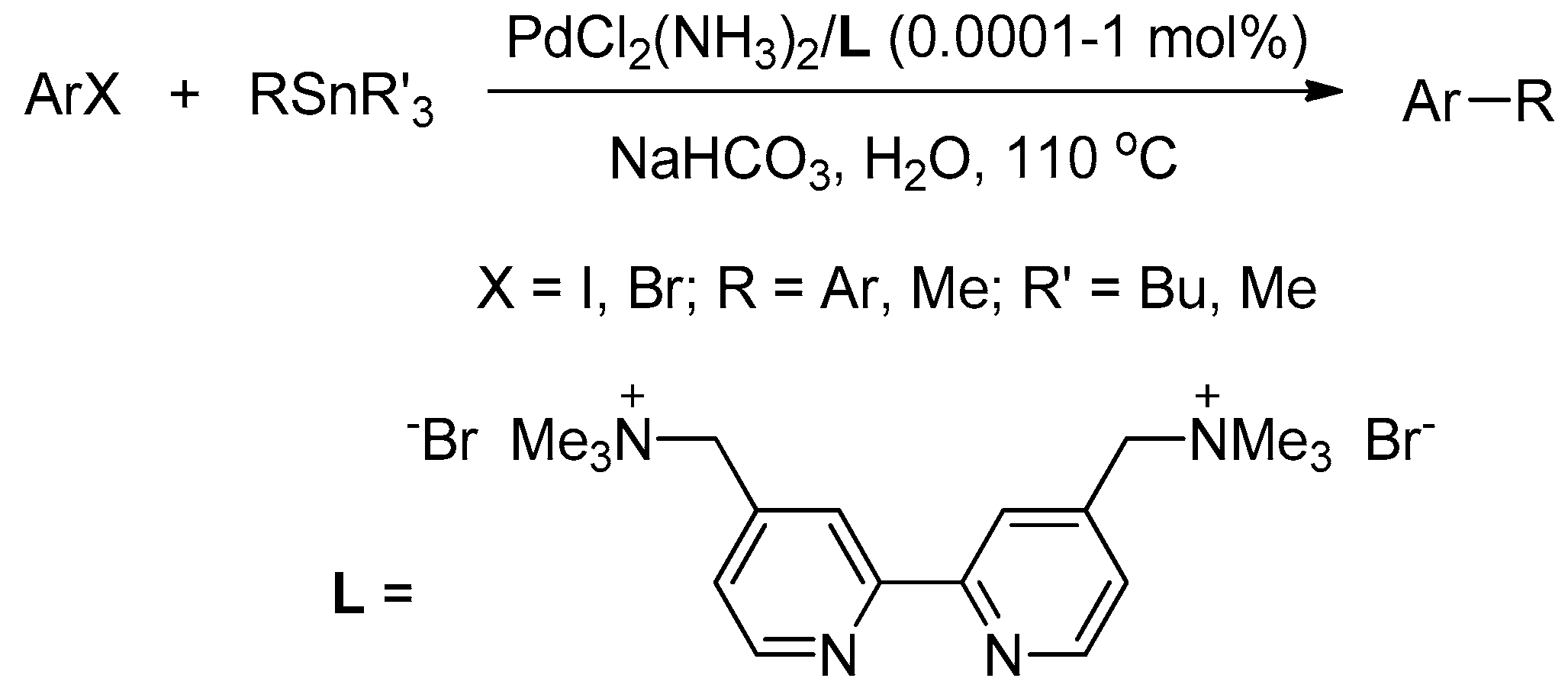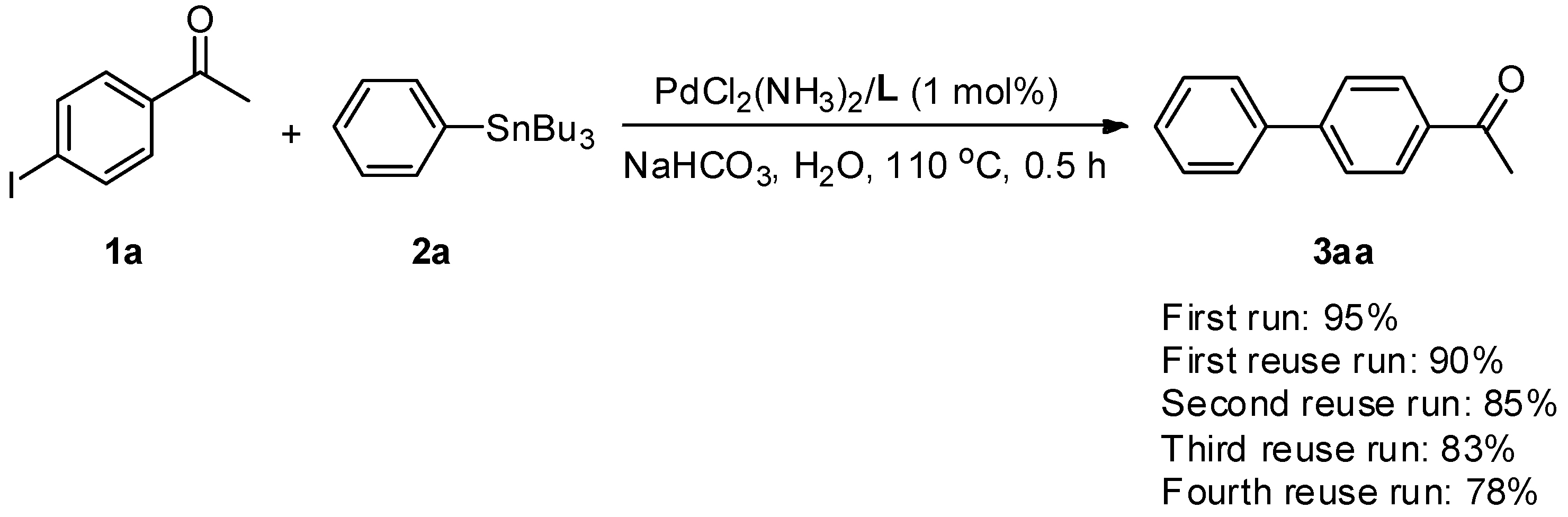Abstract
A water-soluble PdCl2(NH3)2/cationic 2,2′-bipyridyl system was found to be a highly efficient catalyst for Stille coupling of aryl iodides and bromides with organostannanes. The coupling reaction was conducted at 110 °C in water, under aerobic conditions, in the presence of NaHCO3 as a base to afford corresponding Stille coupling products in good to high yields. When aryltributylstannanes were employed, the reactions proceeded smoothly under a very low catalyst loading (as little as 0.0001 mol %). After simple extraction, the residual aqueous phase could be reused in subsequent runs, making this Stille coupling economical. In the case of tetramethylstannane, however, a greater catalyst loading (1 mol %) and the use of tetraethylammonium iodide as a phase-transfer agent were required in order to obtain satisfactory yields.
1. Introduction
The palladium-catalyzed cross-coupling of aryl halides or pseudo-halides with organo-stannanes, known as the Stille coupling, is one of the most powerful methods for the straightforward construction of carbon–carbon bonds in synthetic chemistry [1,2,3]. The main advantages of the Stille coupling reaction include the stability and functional group tolerance of stannanes, the broad reaction scope of aryl halides and pseudo-halides, and its chemoselectivity; therefore, this reaction has been widely applied in natural product synthesis [4,5,6,7,8,9,10], biological research [11], and for pharmaceutical purposes [12]. Stille coupling reactions are generally carried out in organic solvents under homogeneous catalysis, and, hence, it is difficult to separate the catalyst from the reaction mixture and then recycle it at the end of the reaction, leading to wastage of precious metals. Therefore, the development of a recyclable and reusable catalytic system is highly attractive and valuable from the viewpoints of green chemistry and practical application. To circumvent this problem, several new strategies involving heterogenized homogeneous catalysts have been developed for recycling and reusing catalysts, including the use of Pd complexes supported by silica gel [13,14], polymers [15,16], nanoparticles [17,18], porous metal−organic frameworks [19], bulky proazaphosphatrane ligands [20], mesoporous silica [21,22,23,24,25,26], and metal nanoparticles [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Alternatively, combinations of palladium complexes with several green solvents, such as ionic liquids [43,44,45], polyethylene glycol [46,47,48,49,50], H2O [51,52,53,54], or H2O in the presence of surfactants [55,56,57], have also been applied as reusable catalytic systems.
We recently reported that cationic 2,2′-bipyridyl is an excellent ligand to bring PdCl2(NH3)2 into the aqueous phase, in order to efficiently perform several carbon–carbon bond-formation reactions, and the residual aqueous solution can be reused for the next run [58,59,60,61,62,63]. As part of our continuing efforts in the development of water-soluble and reusable catalytic systems for carbon–carbon bond-forming reactions, we report, herein, on a reusable PdCl2(NH3)2/cationic 2,2′-bipyridyl catalytic system, which can be applied for Stille coupling of aryl halides with organostannanes in water under aerobic conditions, in the presence of NaHCO3 as a base. The loading amount of the catalyst in a single batch reaction could be reduced to as little as 0.0001 mol % (1 ppm), still affording products in high yields (Scheme 1).
Scheme 1.
Palladium-catalyzed Stille coupling in water.
2. Results and Discussion
2.1. Optimization of Stille Coupling Conditions
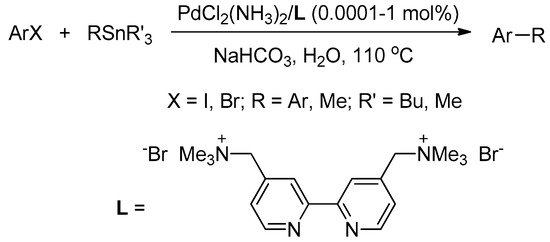
Water-soluble cationic 2,2′-bipyridyl ligand L was synthesized according to our previously-published method [58,59]. The catalytic system was prepared by mixing equimolar amounts of PdCl2(NH3)2 and L in water, and then it was stored under air. Stock solutions of this catalytic system were prepared at different concentrations to obtain various catalyst loadings. In order to discover the optimal conditions, the coupling of 4′-iodoacetophenone (1a, 1 mmol) and PhSnBu3 (2a, 1.2 mmol) in the presence of PdCl2(NH3)2/L (1 mol %) in water (3 mL), at 110 °C for 0.5 h, was first investigated, and the results are summarized in Table 1.

Table 1.
Optimization studies for the Pd(NH3)2Cl2/L-catalyzed Stille coupling of 4′-iodoacetophenone (1a) and PhSnBu3 (2a) in water a.
Initially, several commonly-used bases were screened, and it was found that the use of NaHCO3 provided the Stille coupling product in a 95% yield, which was higher than the yields obtained using other inorganic bases (Entries 1–6). Then, two additional experiments were performed to demonstrate the necessity of water-soluble ligand L. Under the same conditions as Entry 6, only a 32% yield of the cross-coupling product was obtained in the absence of the ligand (Entry 7), and a 35% yield was obtained when L was replaced with neutral 2,2′-bipydryl (Entry 8). These results clearly revealed that use of the water-soluble ligand was crucial in this Stille coupling reaction. When basic aqueous soluble 2,2′-bipyridine-4,4′-dicarboxylic acid was employed as ligand, however, Stille coupling did not occur, hence, 1a and 2a remained intact (Entry 9). Other phenylstannane sources, such as PhSnCl3 and PhSnMe3, were also examined. Although these two reagents furnished 3aa in 90% and 95% yields, respectively (Entries 10 and 11), PhSnBu3 can be synthesized from the much cheaper ClSnBu3; hence, aryltributylstannanes were applied for the reactions.
2.2. Reuse Studies of the Residual Aqueous Solution
We then studied the reusability of the aqueous catalytic system for Stille coupling, which is important from the viewpoints of practical utilization and economics. Coupling of 1a and 2a under the conditions of Entry 6, in Table 1, was performed in order to test the feasibility of this approach (Scheme 2). After completion of the first run, the organic portion was easily separated from the aqueous phase by simple extraction with hexane (3 mL × 3), and 3aa was isolated in a 95% yield using a typical work-up procedure. The residual aqueous solution was then subjected to the next reaction run, charged with the same reactants, 1a and 2a, and NaHCO3. It was found that this residual aqueous solution could be reused at least four times, and a 78% isolated yield was reached in the fourth reuse run. In order to know the partitioning of the catalyst in the organic phase, the first run was performed again. After extracting the reaction mixture with hexane, the organic phase was then used for ICP-MASS analysis. It was found that there was no leaching of Pd into the organic phase. Thus, the slight decrease in activity was presumably due to a gradual decay of the catalytic activity.
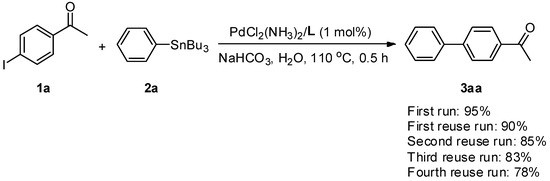
Scheme 2.
Catalyst reuse studies in Stille coupling of 1a and 2a.
2.3. Scope of Substrates and Loading Amounts of Catalyst
Encouraged by the excellent results of the reuse studies of the residual aqueous solution, we then examined the scope of substrates, and attempted to reduce the catalyst loading required (Table 2). Aryl iodides 1a and 1b with electron-withdrawing groups at the para-position coupled with various aryltributylstannanes 2a–2c under 0.01 mol % catalyst loading, giving the corresponding Stille coupling products at yields between 90% and 98%, in 3 h (Entries 1, 3 and 4, 6, and 8 and 9). Further reduction of the catalyst loading to 0.0001 mol % (1 ppm), and increase of the reaction scale to 10 mmol, resulted in the corresponding Stille coupling products being obtained at yields between 72% and 82%, in 48 h (Entries 2, 5, and 7), and the highest turnover number (TON) achieved was up to 820,000 (Entry 2). Iodobenzene (1c) showed only a slightly lower rate compared with electron-withdrawing 1a and 1b, but still resulted in excellent yields by prolonging the reaction time to 6 h (Entries 10–12). For aryl iodides bearing an electron-donating group at the para-position, 1d and 1e, high yields were isolated in 6 h, with a catalyst loading of 0.01 mol % (Entries 13, 15–17, and 19 and 20). Similarly, in the cases of entries 14 and 18, 59% and 66% yields were obtained, respectively, in 48 h, under 1 ppm catalyst loading, using a reaction scale of 10 mmol (Entries 14 and 18).

Table 2.
Pd-catalyzed Stille coupling of aryl iodides (1) and aryltributylstannane (2) in water a.
Analogous reactions of cheaper aryl bromides were also investigated (Table 3). Activated aryl bromides 4a and 4b, were efficiently coupled with 2a–c under conditions identical to those used for aryl iodides; however, a longer reaction time was required (Entries 1–9).

Table 3.
Pd-catalyzed Stille coupling of aryl bromides (4) and aryltributylstannane (2) in water a.
A very low catalyst loading could also be applied when employing electron-withdrawing aryl bromides. For example, the coupling of 4a and 2a furnished 3aa in a 70% yield with a 1 ppm catalyst loading in 48 h (Entry 2). In the cases of 4c and electron-donating 4e, the reactions were much slower than those of the iodide analogs. Hence, conduction of the reaction using a 1 mol % catalyst loading and prolongation of the reaction time were necessary in order to obtain satisfactory yields (Entries 10–15). These results indicated that the oxidative addition of a carbon–bromine bond to palladium may be the rate-determining-step in this catalytic cycle. Surprisingly, the reaction rate was dramatically enhanced when deactivated 4-bromophenol (4f) was employed (Entries 16–20). Compound 4f was soluble in basic aqueous solution, producing 4-bromophenoxide. This aryl bromide then underwent oxidative addition to palladium under homogeneous conditions, making this step much faster than for other water-insoluble aryl bromides. Taking advantage of this water-soluble property, 4f coupled with 2a very smoothly, providing a 56% yield (TON = 560,000) of 3fa under a catalyst loading of only 1 ppm at 110 °C for 48 h (Entry 18).
The utility of this reaction protocol for the formation of Csp2–Csp3 carbon–carbon bonds was also evaluated. As illustrated in Table 4, the coupling of 1a and SnMe4, 5, using 1 mol % catalyst loading at 110 °C for 24 h, gave 6a in only a 51% yield (Entry 1). The use of two equivalents of 5 in the reaction was owing to its low boiling point (74–75 °C). In order to improve upon this outcome, a phase-transfer agent was added into the reaction [55,56,57]. The use of tetrabutylammonium bromide (TBAB) and tetrabutylammonium hydroxide (TBAOH) led to the formation of 6a in yields of 63% and 70%, respectively (Entries 2 and 3). It is worth noting that a 91% isolated yield of 6a could be achieved when tetraethylammonium iodide (TEAI) was applied in the reaction system (Entry 4). Thus, 1b coupled with 5 under such conditions afforded 6b in a 78% yield (Entry 5). However, a low product yield was obtained when electron-rich 1e was utilized (Entry 6). Activated aryl bromides 4a and 4b were also coupled with 5, furnishing 6a and 6b in 40% and 31% yields, respectively (Entries 7 and 8).

Table 4.
Pd-catalyzed Stille coupling of aryl halides (1 or 4) and tetramethylstannane (5) in water a.
3. Experimental Section
3.1. General Information
Chemicals were purchased from commercial suppliers and were used without further purification. Cationic 2,2′-bipyridyl ligand was prepared according to published procedures [58,59]. Aryltributylstannanes were prepared according to known procedures [64]. All 1H- and 13C-NMR spectra were recorded in CDCl3 or DMSO-d6 at 25 °C on a Bruker Biospin AG 300 NMR spectrometer (Bruker Co., Faellanden, Switzerland), in which chemical shifts (δ in ppm) were determined with respect to the non-deuterated solvent as a reference (1H-NMR: CHCl3 at 7.24, non-deuterated DMSO at 2.49 ppm; 13C-NMR: CDCl3 at 77.0, DMSO-d6 at 39.5 ppm). Melting points were recorded using a melting point apparatus, and were uncorrected.
3.2. Typical Stille Coupling Procedure
A sealable tube, equipped with a magnetic stirring bar, was charged with aryl halide (1 mmol), organotin (1.2 mmol), NaHCO3 (2 mmol), and H2O (2 mL). In the case of tetramethyltin, the addition of tetraethylammonium iodide (TEAI, 1 mmol) was required. After the addition of PdCl2(NH3)2/L aqueous solution (1 mL H2O; different concentrations were required for various substrate/catalyst ratios), the tube was sealed under air using a Teflon-coated screw cap. The reaction vessel was then placed in an oil bath at 110 °C for the indicated reaction duration (see Table 2, Table 3 and Table 4). After cooling of the reaction mixture to room temperature, the aqueous solution was extracted with hexane or ethyl acetate; the organic phase was dried over MgSO4, and the solvent was then removed under vacuum. Column chromatography on silica gel afforded the desired product (see Supplementary Materials for the copies of NMR spectra).
4-Phenylacetophenone (3aa, Table 2, Entries 1 and 2, and Table 3, Entries 1 and 2). CAS: 92-91-1; white solid (m.p. = 119–121 °C, lit. [59] 119–121 °C). 1H-NMR (CDCl3): δ 2.62 (s, 3H), 7.39–7.41 (m, 1H), 7.44–7.48 (m, 2H), 7.60–7.63 (m, 2H), 7.66–7.68 (m, 2H), 8.01–8.03 (m, 2H); 13C-NMR (CDCl3): δ 26.6, 127.1(2C), 127.2 (2C), 128.2, 128.8 (2C), 128.9 (2C), 135.7, 139.8, 145.7, 197.7.
4-Acetyl-4′-fluorobiphenyl (3ab, Table 2, Entry 3, and Table 3, Entries 3 and 4). CAS: 720-74-1; white solid (m.p. = 103–104 °C, lit. [59] 103–104 °C). 1H-NMR (CDCl3): δ 2.62 (s, 3H), 7.14 (t, J = 9.0 Hz, 2H), 7.55–7.63 (m, 4H), 7.99–8.02 (m, 2H); 13C-NMR (CDCl3): δ 26.6, 115.9 (d, JC-F = 22.5 Hz, 2C), 127.0 (2C), 128.8 (d, JC-F = 7.5 Hz, 2C), 128.9 (2C), 135.8, 136.0 (d, JC-F = 3.0 Hz), 144.7, 163.0 (d, JC-F = 247.5 Hz), 197.7.
4-Acetyl-4′-methoxybiphenyl (3ac, Table 2, Entries 4 and 5, and Table 3, Entries 5 and 6). CAS: 13021-18-6; pale yellow solid (m.p. = 153–155 °C, lit. [59] 153–155 °C). 1H-NMR (CDCl3): δ 2.60 (s, 3H), 3.84 (s, 3H), 6.98 (d, J = 9.0 Hz, 2H), 7.56 (d, J = 9.0 Hz, 2H), 7.62 (d, J = 9.0 Hz, 2H), 7.99 (d, J = 9.0 Hz, 2H); 13C-NMR (CDCl3): δ 26.6, 55.3, 114.3 (2C), 126.5 (2C), 128.3 (2C), 128.9 (2C), 132.1, 135.2, 145.3, 159.8, 197.7.
4-Phenylbenzonitrile (3ba, Table 2, Entries 6 and 7, and Table 3, Entry 7). CAS: 2920-38-9; white solid (m.p. = 89–91 °C, lit. [65] 89–90 °C). 1H-NMR (CDCl3): δ 7.38–7.50 (m, 3H), 7.55–7.59 (m, 2H), 7.65–7.73 (m, 4H); 13C-NMR (CDCl3): δ 110.8, 118.9, 127.2 (2C), 127.7 (2C), 128.6, 129.1 (2C), 132.6 (2C), 139.1, 145.6.
4-(4-Fluorophenyl)benzonitrile (3bb, Table 2, Entry 8 and Table 3, Entry 8). CAS: 10540-31-5; white solid (m.p. = 115–117 °C, lit. [66] 116–118 °C). 1H-NMR (CDCl3): δ 7.15 (t, J = 9.0 Hz, 2H), 7.52–7.56 (m, 2H), 7.60–7.63 (m, 2H), 7.68–7.61 (m, 2H); 13C-NMR (CDCl3): δ 110.9, 115.9 (d, JC-F = 21.8 Hz, 2C), 118.8, 127.5 (2C), 128.9 (d, JC-F = 8.3 Hz, 2C), 132.6 (2C), 135.2 (d, JC-F =3.0 Hz), 144.6, 163.1 (d, JC-F = 246.8 Hz).
4′-Methoxybiphenyl-4-carbonitrile (3bc, Table 2, Entry 9 and Table 3, Entry 9). CAS: 58743-77-4; pale yellow solid (m.p. = 104–106 °C, lit. [67] 104–105 °C). 1H-NMR (CDCl3): δ 3.84 (s, 3H), 6.99 (d, J = 9.0 Hz, 2H), 7.52 (d, J = 9.0 Hz, 2H), 7.60–7.69 (m, 4H); 13C-NMR (CDCl3): δ 55.4, 100.4 114.5 (2C), 119.1, 127.1 (2C), 128.3 (2C), 131.4, 132.5 (2C), 145.2, 160.2.
Biphenyl (3ca, Table 2, Entry 10 and Table 3, Entry 10). CAS: 92-52-4; white solid (m.p. = 71–72 °C, lit [68] 71–72 °C). 1H-NMR (CDCl3): δ 7.34–7.40 (m, 2H), 7.44–7.49 (m, 4H), 7.61–7.64 (m, 4H); 13C-NMR (CDCl3): δ 127.1 (4C), 127.2(2C), 128.7 (4C), 141.2 (2C).
4-Fluorobiphenyl (3cb, Table 2, Entry 11 and Table 3, Entry 11). CAS: 324-74-3; white solid (m.p. = 72–73 °C, lit. [69] 72–73 °C). 1H-NMR (CDCl3): δ 7.10–7.15 (m, 2H), 7.34–7.37 (m,1H), 7.41–7.46 (m, 2H), 7.52–7.61 (m, 4H); 13C-NMR (CDCl3): δ 115.6 (d, JC-F = 21.0 Hz, 2C), 127.0 (2C), 127.2, 128.7 (d, JC-F = 8.3 Hz, 2C), 128.8 (2C), 137.3 (d, JC-F = 3.0 Hz), 140.2, 162.4 (d, JC-F = 245.3 Hz).
4-Methoxybiphenyl (3cc, Table 2, Entries 12, 17 and 18, and Table 3, Entries 12 and 13). CAS: 613-37-6; white solid (m.p. = 85–87 °C, lit. [59] 85–87 °C). 1H-NMR (CDCl3): δ 3.85 (s, 3H), 6.99 (d, J = 9.0 Hz, 2H), 7.31–7.34 (m, 1H), 7.40–7.45 (m, 2H), 7.52–7.58 (m, 4H); 13C-NMR (CDCl3): δ 55.3, 114.2 (2C), 126.6, 126.7 (2C), 128.1 (2C), 128.7 (2C), 133.7, 140.8, 159.1.
4-Methylbiphenyl (3da, Table 2, Entries 13 and 14). CAS: 644-08-6; white solid (m.p. = 43–45 °C, lit. [59] 43–45 °C). 1H-NMR (CDCl3): δ 2.41 (s, 3H), 7.24–7.28 (m, 2H), 7.34–7.36 (m, 1H), 7.42–7.47 (m, 2H), 7.50–7.53 (m, 2H), 7.58–7.61 (m, 2H); 13C-NMR (CDCl3): δ 21.1, 126.8, 126.9 (4C), 128.7 (2C), 129.5 (2C), 137.0, 138.3, 141.1.
4-Fluoro-4′-methylbiphenyl (3db, Table 2, Entry 15). CAS: 72093-43-7; white solid (m.p. = 79–81 °C, lit. [70] 78–79 °C). 1H-NMR (CDCl3): δ 2.39 (s, 3H), 7.11 (t, J = 9.0 Hz, 2H), 7.23–7.25 (m, 2H), 7.42–7.45 (m, 2H), 7.50–7.54 (m, 2H); 13C-NMR (CDCl3): δ 21.1, 115.6 (d, JC-F = 21.8 Hz, 2C), 126.8 (2C), 128.5 (d, JC-F = 8.3 Hz, 2C), 129.5 (2C), 137.0, 137.2 (d, JC-F = 3.0 Hz), 137.4, 162.3 (d, JC-F = 247.5 Hz).
4-Methoxy-4′-methylbiphenyl (3dc, Table 2, Entry 16). CAS: 53040-92-9; white solid (m.p. = 113–114 °C, lit. [59] 113–114 °C). 1H-NMR (CDCl3): δ 2.42 (s, 3H), 3.87 (s, 3H), 7.01 (d, J = 9.0 Hz, 2H), 7.27 (d, J = 9.0 Hz, 2H), 7.50 (d, J = 9.0 Hz, 2H), 7.56 (d, J = 9.0 Hz, 2H); 13C-NMR (CDCl3): δ 21.0, 55.2, 114.1 (2C), 126.5 (2C), 127.9 (2C), 129.4 (2C), 133.6, 136.3, 137.9, 158.9.
4-Fluoro-4′-methoxybiphenyl (3eb, Table 2, Entry 19 and Table 3, Entry 14). CAS: 450-39-5; white solid (m.p. = 88–90 °C, lit. [59] 88–90 °C). 1H-NMR (CDCl3): δ 3.84 (s, 3H), 6.94–6.99 (m, 2H), 7.07–7.13 (m, 2H), 7.44–7.51 (m, 4H); 13C-NMR (CDCl3): δ 55.3, 114.2 (2C), 115.5 (d, JC-F = 21.8 Hz, 2C), 128.0 (2C), 128.2 (d, JC-F = 7.5 Hz, 2C), 132.8, 136.9 (d, JC-F = 3.0 Hz), 159.1, 162.0 (d, JC-F = 243.8 Hz).
4,4′-Dimethoxybiphenyl (3ec, Table 2, Entry 20 and Table 3, Entry 15). CAS: 2132-80-1; pale yellow solid (m.p. = 178–180 °C, lit. [68] 178–180 °C). 1H-NMR (CDCl3): δ 3.84 (s, 6H), 6.93–6.97 (m, 4H), 7.45–7.49 (m, 4H); 13C-NMR (CDCl3): δ 55.3 (2C), 114.1 (4C), 127.7 (4C), 133.4 (2C), 158.6 (2C).
4-Phenylphenol (3fa, Table 3, Entries 16–18). CAS: 92-69-3; white solid (m.p. = 148–150 °C, lit. [59] 148–150 °C). 1H-NMR (DMSO–d6): δ 6.86 (d, J = 9.0 Hz, 2H), 7.25–7.28 (m, 1H), 7.36–7.57 (m, 6H), 9.57 (br, 1H); 13C-NMR (DMSO–d6): δ 115.7 (2C), 126.0 (2C), 126.4, 127.8 (2C), 128.8 (2C), 130.9, 140.2, 157.2.
4-Hydroxy-4-fluorobiphenyl (3fb, Table 3, Entry 19). CAS: 324-94-7; white solid (m.p. = 168–170 °C, lit. [71] 167–170 °C). 1H-NMR (DMSO–d6): δ 6.84 (d, J = 9.0 Hz, 2H), 7.21 (t, J = 9.0 Hz, 2H), 7.44 (d, J = 9.0 Hz, 2H), 7.55–7.60 (m, 2H), 9.56 (br, 1H); 13C-NMR (DMSO–d6): δ 115.5 (d, JC-F = 21.0 Hz, 2C), 115.7 (2C), 127.7 (2C), 127.8 (d, JC-F = 9.0 Hz, 2C), 130.0, 136.7 (d, JC-F = 3.0 Hz), 157.1, 161.2 (d, JC-F = 240.8 Hz).
4-Hydroxy-4-methoxybiphenyl (3fc, Table 3, Entry 20). CAS: 16881-71-3; white solid (m.p. = 173–175 °C, lit. [72] 171–173 °C). 1H-NMR (DMSO–d6): δ 3.75 (s, 3H), 6.82 (d, J = 9.0 Hz, 2H), 6.95 (d, J =9.0 Hz, 2H), 7.40 (d, J = 9.0 Hz, 2H), 7.48 (d, J = 9.0 Hz, 2H), 9.46 (br, 1H); 13C-NMR (DMSO–d6): δ 55.1, 114.2 (2C), 115.7 (2C), 127.0 (2C), 127.2 (2C), 130.7, 132.8, 156.5, 158.1.
4′-Methylacetophenone (6a [73], Table 4, Entries 1–4 and 7). CAS: 122-00-9; colorless liquid. 1H-NMR (CDCl3): δ 2.38 (s, 3H), 2.55 (s, 3H), 7.23 (d, J = 9.0 Hz, 2H), 7.83 (d, J = 8.3 Hz, 2H); 13C-NMR (CDCl3): δ 21.6, 26.5, 128.4 (2C), 129.2 (2C), 134.6, 143.8, 197.8.
p-Tolunitrile (6b [74], Table 4, Entries 5 and 8). CAS: 104-85-8; colorless liquid. 1H-NMR (CDCl3): δ 2.39 (s, 3H), 7.24 (d, J = 9.0 Hz, 2H), 7.51 (d, J = 9.0 Hz, 2H); 13C-NMR (CDCl3): δ 21.8, 109.2, 119.1, 129.8 (2C), 131.9 (2C), 143.6.
4-Methylanisole (6e [26], Table 4, Entry 6). CAS: 104-93-8; colorless liquid. 1H-NMR (CDCl3): δ 2.30 (s, 3H), 3.79 (s, 3H), 6.82 (d, J = 9.0 Hz, 2H), 7.10 (d, J = 9.0 Hz, 2H); 13C-NMR (CDCl3): δ 20.4, 55.2, 113.6 (2C), 129.7, 129.8 (2C), 157.4.
3.3. Procedure for Reuse of the Catalytic Aqueous Solution
The reaction was conducted following the procedure described in Section 3.2., and under the reaction conditions shown in Table 1, Entry 6. After the initial reaction, the aqueous reaction mixture was extracted with hexane (3 mL × 3) under vigorous stirring. The organic layer was separated from the aqueous phase by syringe, and the organic product was isolated from the combined organic phase according to a previously-described in Section 3.2. The residual aqueous solution was then charged with 1a, 2a, and NaHCO3 for the next reaction.
4. Conclusions
In conclusion, we have proved that the PdCl2(NH3)2/cationic 2,2′-bipyridyl system is highly efficient and provides a reusable catalyst for Stille couplings. This catalytic system exhibits a high efficiency for the coupling of aryl iodides and activated aryl bromides with various aryltributylstannanes under a very low catalyst loading (1 ppm). This water-compatible catalytic system enables the reaction to be conducted by a very simple procedure, allowing easy separation of the catalytic aqueous solution from the organic products, rendering it very suitable for practical applications.
Supplementary Materials
The following are available online at: http://www.mdpi.com/1420-3049/21/9/1025/s1: copies of 1H- and 13C-NMR spectra of all Stille coupling products.
Acknowledgments
This research was financially supported by the Ministry of Science and Technology of Taiwan (MOST 104-2113-M-027-003). We thank Yi-Tsu Chan (National Taiwan University) for performing ICP-MASS analysis.
Author Contributions
Wei-Yi Wu and Fu-Yu Tsai conceived and designed the research; Ling-Jun Liu, Fen-Ping Chang, and Yu-Lun Cheng performed the experiments; Fu-Yu Tsai wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stille, J.K. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles [new synthetic methods (58)]. Angew. Chem. Int. Ed. 1986, 25, 508–524. [Google Scholar] [CrossRef]
- Espinet, P.; Echavarren, A.M. The mechanisms of the Stille reaction. Angew. Chem. Int. Ed. 2004, 43, 4704–4734. [Google Scholar]
- Cordovilla, C.; Bartolomé, C.; Martínez-Ilarduya, J.M.; Espinet, P. The Stille reaction, 38 years later. ACS Catal. 2015, 5, 3040–3053. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Chakraborty, T.K.; Piscopio, A.D.; Minowa, N.; Bertinato, P. Total synthesis of rapamycin. J. Am. Chem. Soc. 1993, 115, 4419–4420. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Piscopio, A.D.; Bertinato, P.; Chakraborty, T.K.; Minowa, N.; Koide, K. Total synthesis of rapamycin. Chem. Eur. J. 1995, 1, 318–333. [Google Scholar] [CrossRef]
- Shair, M.D.; Yoon, T.Y.; Mosny, K.K.; Chou, T.C.; Danishefsky, S.J. The total synthesis of dynemicin A leading to development of a fully contained bioreductively activated enediyne prodrug. J. Am. Chem. Soc. 1996, 118, 9509–9525. [Google Scholar] [CrossRef]
- Amans, D.; Bareille, L.; Bellosta, V.; Cossy, J. Synthesis of the monomeric counterpart of marinomycin A. J. Org. Chem. 2009, 74, 7665–7674. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, W.P.; Gallagher, K.A.; Jean, M.; Schmidt, J.P.; Diorazio, L.J.; Taylor, R.J.K. Direct imine acylation: Synthesis of the proposed structures of ‘upenamide. Org. Lett. 2013, 15, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Souris, C.; Frébault, F.; Patel, A.; Audisio, D.; Houk, K.N.; Maulide, N. Stereoselective synthesis of dienyl-carboxylate building blocks: Formal synthesis of inthomycin C. Org. Lett. 2013, 15, 3242–3245. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Hashemi, E.; Azimian, F. Recent developments of the Stille reaction as a revolutionized method in total synthesis. Tetrahedron 2014, 70, 7–21. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; King, N.P.; Finlay, M.R.V.; He, Y.; Roschangar, F.; Vourloumis, D.; Vallberg, H.; Sarabia, F.; Ninkovic, S.; Hepworth, D. Total synthesis of epothilone E and related side-chain modified analogues via a Stille coupling based strategy. Bioorg. Med. Chem. 1999, 7, 665–697. [Google Scholar] [CrossRef]
- Ragan, J.A.; Raggon, J.W.; Hill, P.D.; Jones, B.P.; McDermott, R.E.; Munchhof, M.J.; Marx, M.A.; Casavant, J.M.; Copper, B.A.; Doty, J.L.; Lu, Y. Cross-coupling methods for the large-scale preparation of an imidazole-thienopyridine: Synthesis of [2-(3-methyl-3H-imidazol-4-yl)-thieno[3,2-b]pyridin-7-yl]-(2-methyl-1H-indol-5-yl)-amine. Org. Proc. Res. Dev. 2003, 7, 676–683. [Google Scholar] [CrossRef]
- Lee, D.-H.; Jung, J.-Y.; Jin, M.-J. Highly active and recyclable silica gel-supported palladium catalyst for mild cross-coupling reactions of unactivated heteroaryl chlorides. Green Chem. 2010, 12, 2024–2029. [Google Scholar] [CrossRef]
- McAfee, S.M.; McCahill, J.S.J.; Macaulay, C.M.; Hendsbee, A.D.; Welch, G.C. Utility of a heterogeneous palladium catalyst for the synthesis of a molecular semiconductor via Stille, Suzuki, and direct heteroarylation cross-coupling reactions. RSC Adv. 2015, 5, 26097–26106. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Lofù, A.; Mastrorilli, P.; Mucciante, V.; Nobile, C.F. Stille coupling reactions catalysed by a polymer supported palladium complex. J. Organomet. Chem. 2006, 691, 131–137. [Google Scholar] [CrossRef]
- Bahari, S.; Mohammadi-Aghdam, B.; Molaei, R.; Gharibi, Z. Diphenylphosphinoethane-functionalized polystyrene resin-supported Pd(0) complex—A highly active and recyclable catalyst for the Stille reaction under aerobic conditions. Can. J. Chem. 2012, 90, 784–789. [Google Scholar] [CrossRef]
- Jin, M.-J.; Lee, D.-H. A practical heterogeneous catalyst for the Suzuki, Sonogashira, and Stille coupling reactions of unreactive aryl chlorides. Angew. Chem. Int. Ed. 2010, 49, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani-Choghamarani, A.; Norouzi, M. Palladium supported on modified magnetic nanoparticles: A phosphine-free and heterogeneous catalyst for Suzuki and Stille reactions. Appl. Organomet. Chem. 2016, 30, 140–147. [Google Scholar] [CrossRef]
- Saha, D.; Sen, R.; Maity, T.; Koner, S. Anchoring of palladium onto surface of porous metal−organic framework through post-synthesis modification and studies on Suzuki and Stille coupling reactions under heterogeneous condition. Langmuir 2013, 29, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Urgaonkar, S.; McLaughlin, P.A.; Verkade, J.G. Highly active palladium catalysts supported by bulky proazaphosphatrane ligands for Stille cross-coupling: Coupling of aryl and vinyl chlorides, room temperature coupling of aryl bromides, coupling of aryl triflates, and synthesis of sterically hindered biaryls. J. Am. Chem. Soc. 2004, 126, 16433–16439. [Google Scholar] [PubMed]
- Zhao, H.; Wang, Y.; Sha, J.; Sheng, S.; Cai, M. MCM-41-supported bidentate phosphine palladium(0) complex as an efficient catalyst for the heterogeneous Stille reaction. Tetrahedron 2008, 64, 7517–7523. [Google Scholar] [CrossRef]
- Jana, S.; Haldar, S.; Koner, S. Heterogeneous Suzuki and Stille coupling reactions using highly efficient palladium(0) immobilized MCM-41 catalyst. Tetrahedron Lett. 2009, 50, 4820–4823. [Google Scholar] [CrossRef]
- Cai, M.; Zheng, G.; Ding, G. The first heterogeneous carbonylative Stille coupling of organostannanes with aryl iodides catalyzed by MCM-41-supported bidentate phosphine palladium(0) complex. Green Chem. 2009, 11, 1687–1693. [Google Scholar] [CrossRef]
- Bhunia, S.; Koner, S. Heterogeneous Stille and Sonogashira cross-coupling reactions over palladium anchored mesoporous silica catalyst. Indian J. Chem. Sect. A 2011, 50, 1380–1387. [Google Scholar]
- Ghorbani-Choghamarani, A.; Nikpour, F.; Ghorbani, F.; Havasi, F. Anchoring of Pd(II) complex in functionalized MCM-41 as an efficient and recoverable novel nano catalyst in C–C, C–O and C–N coupling reactions using Ph3SnCl. RSC Adv. 2015, 5, 33212–33220. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, G.; Hao, W.; Cai, M. MCM-41-supported mercapto palladium(0) complex: An efficient and recyclable catalyst for the heterogeneous Stille coupling reaction. Appl. Organomet. Chem. 2010, 24, 92–98. [Google Scholar] [CrossRef]
- Choudary, B.M.; Madhi, S.; Chowdari, N.S.; Kantam, M.L.; Sreedhar, B. Layered double hydroxide supported nanopalladium catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-type coupling reactions of chloroarenes. J. Am. Chem. Soc. 2002, 124, 14127–14136. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, J.C.; Lezutekong, R.; Crooks, R.M. Dendrimer-encapsulated Pd nanoparticles as aqueous, room-temperature catalysts for the Stille reaction. J. Am. Chem. Soc. 2005, 127, 5097–5103. [Google Scholar] [CrossRef] [PubMed]
- Calò, V.; Nacci, A.; Monopoli, A.; Montingelli, F. Pd nanoparticles as efficient catalysts for Suzuki and Stille coupling reactions of aryl halides in ionic liquids. J. Org. Chem. 2005, 70, 6040–6044. [Google Scholar] [CrossRef] [PubMed]
- Tatumi, R.; Akita, T.; Fujihara, H. Synthesis of small palladium nanoparticles stabilized by bisphosphine BINAP bearing an alkyl chain and their palladium nanoparticle–catalyzed carbon–carbon coupling reactions under room-temperature. Chem. Commun. 2006, 3349–3351. [Google Scholar] [CrossRef] [PubMed]
- Pacardo, D.B.; Sethi, M.; Jones, S.E.; Naik, R.R.; Knecht, M.R. Biomimetic synthesis of Pd nanocatalysts for the Stille coupling reaction. ACS Nano 2009, 3, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Sawoo, S.; Srimani, D.; Dutta, P.; Lahiri, R.; Sarkar, A. Size controlled synthesis of Pd nanoparticles in water and their catalytic application in C–C coupling reactions. Tetrahedron 2009, 65, 4367–4374. [Google Scholar] [CrossRef]
- Bernechea, M.; de Jesús, E.; López-Mardomingo, C.; Terreros, P. Dendrimer-encapsulated Pd nanoparticles versus palladium acetate as catalytic precursors in the Stille reaction in water. Inorg. Chem. 2009, 48, 4491–4496. [Google Scholar] [CrossRef] [PubMed]
- Pacardo, D.B.; Slocik, J.M.; Kirk, K.C.; Naik, R.R.; Knecht, M.R. Interrogating the catalytic mechanism of nanoparticle mediated Stille coupling reactions employing bio-inspired Pd nanocatalysts. Nanoscale 2011, 3, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Sarkar, A. Palladium nanoparticles immobilized on chemically modified silica gel: Efficient heterogeneous catalyst for Suzuki, Stille and Sonogashira cross-coupling reactions. Adv. Synth. Catal. 2011, 353, 2814–2822. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Tao, Z. A mild and recyclable nano-sized nickel catalyst for the Stille reaction in water. Catal. Sci. Technol. 2012, 2, 707–710. [Google Scholar] [CrossRef]
- Pacardo, D.B.; Knecht, M.R. Exploring the mechanism of Stille C–C coupling via peptide-capped Pd nanoparticles results in low temperature reagent selectivity. Catal. Sci. Technol. 2013, 3, 745–753. [Google Scholar] [CrossRef]
- Yano, H.; Nakajima, Y.; Obora, Y. N,N-Dimethylformamide-stabilized palladium nanoclusters as catalyst for Migita-Kosugi-Sstille cross-coupling reactions. J. Organomet. Chem. 2013, 745–746, 258–261. [Google Scholar] [CrossRef]
- Sun, J.; Bao, M.; Feng, X.; Yu, X.; Yamamoto, Y.; Almansour, A.I.; Arumugam, N.; Kumar, R.S. Carboxylative coupling reaction of five-membered (chloromethyl) heteroarenes with allyltributylstannane catalyzed by palladium nanoparticles. Tetrahedron Lett. 2015, 56, 6747–6750. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Wang, K.; Fu, Y.; Du, Z. Microwave-assisted Stille cross-coupling reaction catalysed by in situ formed palladium nanoparticles. J. Chem. Res. 2015, 39, 399–402. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Honarmand, E.; Maham, M. Preparation of palladium nanoparticles using Euphorbia thymifolia L. leaf extract and evaluation of catalytic activity in the ligand-free Stille and Hiyama cross-coupling reactions in water. New J. Chem. 2015, 39, 4745–4752. [Google Scholar] [CrossRef]
- Li, X.; Zhu, T.; Shao, Z.; Li, Y.; Chang, H.; Gao, W.; Zhang, Y.; Wei, W. Newly-generated Al(OH)3-supported Pd nanoparticles-catalyzed Stille and Kumada coupling reactions of diazonium salts, (Het)aryl chlorides. Tetrahedron 2016, 72, 69–75. [Google Scholar] [CrossRef]
- Handy, S.T.; Zhang, X. Organic synthesis in ionic liquids: The Stille coupling. Org. Lett. 2001, 3, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Chiappe, C.; Imperato, G.; Napolitano, E.; Pieraccini, D. Ligandless Stille cross-coupling in ionic liquids. Green Chem. 2004, 6, 33–36. [Google Scholar] [CrossRef]
- Hao, W.; Xi, Z.; Cai, M. A practical synthesis of biaryls and aromatic acetylenes by Stille coupling in room-temperature ionic liquids. Synth. Commun. 2012, 42, 2396–2406. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Wang, K.-H.; Wang, J.-X. Atom-efficient, palladium-catalyzed Stille coupling reactions of tetraphenylstannane with aryl iodides or aryl bromides in polyethylene glycol 400 (PEG-400). Adv. Synth. Catal. 2009, 351, 1378–1382. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Wang, K.-H.; Wang, J.-X. Pd(PPh3)4-PEG 400 catalyzed protocol for the atom-efficient Stille cross-coupling reaction of organotin with aryl bromides. J. Org. Chem. 2009, 74, 5599–5602. [Google Scholar] [CrossRef] [PubMed]
- Iranpoor, N.; Firouzabadi, H.; Davan, E.E.; Rostami, A.; Nematollahi, A. Triphenyltin chloride as a new source of phenyl group for C-heteroatom and C-C bond formation. J. Organomet. Chem. 2013, 740, 123–130. [Google Scholar] [CrossRef]
- Naghipour, A.; Ghorbani-Choghamarani, A.; Heidarizadi, F.; Notash, B. Synthesis, characterization and structural study of a phosphonium salt containing the [Pd2Br6]2− ion and its application as a novel, efficient and renewable heterogeneous catalyst for amination of aryl halides and the Stille cross-coupling reaction. Polyhedron 2016, 105, 18–26. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Naghipour, A.; Heidarizadi, F.; Shirkhani, R.; Notash, B. Bis[(2-methylacetatobenzyl)tri(p-tolyl)phosphonium] hexabromodipalladate(II); synthesis, characterization, structural study and application as a retrievable heterogeneous catalyst for the amination of aryl halides and Stille cross-coupling reaction. Inorg. Chim. Acta 2016, 446, 97–102. [Google Scholar] [CrossRef]
- Roshchin, A.I.; Bumagin, N.A.; Beletskaya, I.P. Palladium-catalyzed cross-coupling reaction of organostannoates with aryl halides in aqueous medium. Tetrahedron Lett. 1995, 36, 125–128. [Google Scholar] [CrossRef]
- Rai, R.; Aubrecht, K.B.; Collum, D.B. Palladium-catalyzed Stille couplings of aryl-, vinyl-, and alkyltrichlorostannanes in aqueous solution. Tetrahedron Lett. 1995, 36, 3111–3114. [Google Scholar] [CrossRef]
- Wolf, C.; Lerebours, R. Efficient Stille cross-coupling reaction using aryl chlorides or bromides in water. J. Org. Chem. 2003, 68, 7551–7554. [Google Scholar] [CrossRef] [PubMed]
- Ogo, S.; Takebe, Y.; Uehara, K.; Yamazaki, T.; Nakai, H.; Watanabe, Y.; Fukuzumi, S. pH-Dependent C-C coupling reactions catalyzed by water-soluble palladacyclic aqua catalysts in water. Organometallics 2006, 25, 331–338. [Google Scholar] [CrossRef]
- Li, J.-H.; Hu, X.-C.; Liang, Y.; Xie, Y.-X. PEG-400 promoted Pd(OAc)2/DABCO-catalyzed cross-coupling reactions in aqueous media. Tetrahedron 2006, 62, 31–38. [Google Scholar] [CrossRef]
- Susanto, W.; Chu, C.-Y.; Ang, W.J.; Chou, T.-C.; Lo, L.-C.; Lam, Y. Fluorous oxime palladacycle: A precatalyst for carbon−carbon coupling reactions in aqueous and organic medium. J. Org. Chem. 2012, 77, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.-P.; Cai, C.; Lipshutz, B.H. Stille couplings in water at room temperature. Green Chem. 2013, 15, 105–109. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Chen, S.-N.; Tsai, F.-Y. Recyclable and highly active cationic 2,2′-bipyridyl palladium(II) catalyst for Suzuki cross-coupling reaction in water. Tetrahedron Lett. 2006, 47, 9267–9270. [Google Scholar] [CrossRef]
- Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Hiyama reaction of aryl bromides with arylsiloxanes catalyzed by a reusable palladium(II)/cationic bipyridyl system in water. Tetrahedron 2008, 64, 8164–8168. [Google Scholar] [CrossRef]
- Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Homocoupling reaction of terminal alkynes catalyzed by a reusable cationic 2,2′-bipyridyl palladium(II)/CuI system in water. Green Chem. 2009, 11, 269–274. [Google Scholar] [CrossRef]
- Huang, S.-H.; Wu, T.-M.; Tsai, F.-Y. pH-Dependent conjugate addition of arylboronic acids to α,β-unsaturated enones catalyzed by a reusable palladium(II)/cationic 2,2′-bipyridyl system in water under air. Appl. Organomet. Chem. 2010, 24, 619–624. [Google Scholar] [CrossRef]
- Huang, S.-H.; Chen, J.-R.; Tsai, F.-Y. Palladium(II)/cationic 2,2′-bipyridyl system as a highly efficient and reusable catalyst for the Mizoroki-Heck reaction in water. Molecules 2010, 15, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Huang, S.-H.; Lee, H.-S.; Tsai, F.-Y. A reusable palladium(II)/cationic 2,2′-bipyridyl catalytic system for hydroxycarbonylation of aryl iodides in water. J. Chin. Chem. Soc. 2013, 60, 769–772. [Google Scholar] [CrossRef]
- Ren, Y.; Dienes, Y.; Hettel, S.; Parvez, M.; Hoge, B.; Baumgartner, T. Highly fluorinated dithieno[3,2-b:2′,3′-d]phospholes with stabilized LUMO levels. Organometallics 2009, 28, 734–740. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Karami, K.; Pirisedigh, A. Application of dimeric orthopalladated complex in Suzuki–Miyaura cross coupling reaction under microwave irradiation and conventional heating. Inorg. Chim. Acta 2011, 370, 531–535. [Google Scholar] [CrossRef]
- Bernhardt, S.; Manolikakes, G.; Kunz, T.; Knochel, P. Preparation of solid salt-stabilized functionalized organozinc compounds and their application to cross-coupling and carbonyl addition reactions. Angew. Chem. Int. Ed. 2011, 50, 9205–9209. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhou, W.; Wang, F.; Wang, J.-X. In situ generation of palladium nanoparticles: Ligand-free palladium catalyzed ultrafast Suzuki-Miyaura cross-coupling reaction in aqueous phase at room temperature. Tetrahedron 2011, 67, 4914–4918. [Google Scholar] [CrossRef]
- Tsai, F.-Y.; Lin, B.-N.; Chen, M.-J.; Mou, C.-Y.; Liu, S.-T. Anchored palladium bipyridyl complex in nanosized MCM-41: A recyclable and efficient catalyst for the Kumada–Corriu reaction. Tetrahedron 2007, 63, 4304–4309. [Google Scholar] [CrossRef]
- Worm-Leonhard, K.; Meldal, M. Green catalysts: Solid-phase peptide carbene ligands in aqueous transition-metal catalysis. Eur. J. Org. Chem. 2008, 5244–5253. [Google Scholar] [CrossRef]
- Kuriyama, M.; Shimazawa, R.; Shirai, R. Design and synthesis of thioether-imidazolium chlorides as efficient ligands for palladium-catalyzed Suzuki–Miyaura coupling of aryl bromides with arylboronic acids. Tetrahedron 2007, 63, 9393–9400. [Google Scholar] [CrossRef]
- Schmidt, B.; Hölter, F. Suzuki–Miyaura cross coupling reactions with phenoldiazonium salts. Org. Biomol. Chem. 2011, 9, 4914–4920. [Google Scholar] [CrossRef] [PubMed]
- DeSchepper, R.E.; Swenton, J.S. Anodic oxidation studies of oxygenated biphenyls. Convenient synthetic routes to certain functionalized biphenyls. Tetrahedron Lett. 1985, 26, 4831–4834. [Google Scholar] [CrossRef]
- Cunningham, A.; Mokal-Parekh, V.; Wilson, C.; Woodward, S. On the use of mixtures of organotin species for catalytic enantioselective ketone allylation—A detective story. Org. Biomol. Chem. 2004, 2, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.-C.; Huang, K.-S.; Chen, H.-M.; Wu, C.-C.; Lin, G.-J. An efficient method for the preparation of nitriles via the dehydration of aldoximes with phthalic anhydride. J. Chin. Chem. Soc. 2004, 51, 619–627. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 3 and 6 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).