Hair Growth Promoting and Anticancer Effects of p21-activated kinase 1 (PAK1) Inhibitors Isolated from Different Parts of Alpinia zerumbet
Abstract
:1. Introduction
2. Results
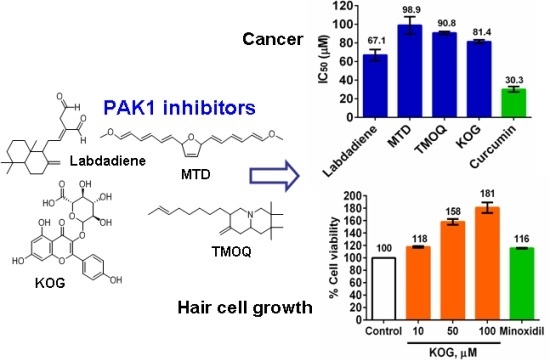
2.1. Effect of Extract and Isolated Compounds on Hair Cell Growth
2.2. Anticancer Activity
2.3. Direct Inhibition of PAK1 in Vitro
3. Discussion
4. Materials and Methods
4.1. Chemicals and Instrumental Analysis
4.2. Preparation of the Extracts and Isolation of Compounds
4.3. Assay for in Vitro Hair Cell Growth Promotion
4.4. Anticancer Activity by MTT Assay
4.5. In Vitro Assay for PAK1 Inhibition
4.6. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kawano, M.; Han, J.; Kchouk, M.E.; Isoda, H. Hair growth regulation by the extract of aromatic plant Erica multiflora. J. Nat. Med. 2009, 63, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Junlatat, J.; Sripanidkulchai, B. Hair growth-promoting effect of Carthamus tinctorius floret extract. Phytother. Res. 2014, 28, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- McElwee, K.J.; Sinclair, R. Hair physiology and its disorders. Drug Discov. Today Dis. Mech. 2008, 5, 163–171. [Google Scholar] [CrossRef]
- Sun, Y.N.; Cui, L.; Li, W.; Yan, X.T.; Yang, S.Y.; Kang, J.I.; Kang, H.K.; Kim, Y.H. Promotion effect of constituents from the root of Polygonum multiflorum on hair growth. Bioorg. Med. Chem. Lett. 2013, 23, 4801–4805. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hao, H.; Xia, L.; Liu, J.; Ti, D.; Tong, C.; Hou, Q.; Han, Q.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Kim, S.C.; Kim, M.K.; Boo, H.J.; Jeon, Y.J.; Koh, Y.S.; Yoo, E.S.; Kang, S.M.; Kang, H.K. Effect of dieckol, a component of Ecklonia cava, on the promotion of hair growth. Int. J. Mol. Sci. 2012, 13, 6407–6423. [Google Scholar] [CrossRef] [PubMed]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [PubMed]
- Zhang, N.N.; Park, D.K.; Park, H.J. Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement. Altern. Med. 2013, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.N.; Poornachandra, Y.; Nagender, P.; Kumar, G.S.; Swaroop, D.K.; Kumar, C.G.; Narsaiah, B. Synthesis of novel nicotinohydrazide and (1,3,4-oxadiazol-2-yl)-6-(trifluoromethyl)pyridine derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4829–4831. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, S.L.; Hussein, M.M.; Abdel Rahman, D.E.; Abdel Ghany, L.M. Synthesis, docking and in vitro anticancer evaluation of some new benzopyrone derivatives. Bioorg. Chem. 2014, 53, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, H.R.; Park, E.; Lee, S.C. Cytotoxic and antitumor activity of momilactone B from rice hulls. J. Agric. Food Chem. 2007, 55, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Sharma, V.; Chauhan, N.S.; Thakur, M.; Dixit, V.K. Evaluation of hair growth promoting activity of Phyllanthus niruri. Avicenna J. Phytomed. 2015, 5, 512–519. [Google Scholar] [PubMed]
- Maruta, H. Herbal therapeutics that block the oncogenic kinase PAK1: A practical approach towards PAK1-dependent diseases and longevity. Phytother. Res. 2014, 28, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Messerli, S.M.; Ahn, M.R.; Kunimasa, K.; Yanagihara, M.; Tatefuji, T; Hashimoto, K.; Mautner, V.; Uto, Y.; Hori, H.; Kumazawa, S.; et al. Artepillin C (ARC) in Brazilian green propolis selectively blocks oncogenic PAK1 signaling and suppresses the growth of NF tumors in mice. Phytother. Res. 2009, 23, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Be Tu, P.T.; Nguyen, B.C.Q.; Tawata, S.; Yun, C.Y.; Kim, E.G.; Maruta, H. The serum/PDGF-dependent “melanogenic” role of the minute level of the oncogenic kinase PAK1 in melanoma cells proven by the highly sensitive kinase assay. Drug Discov. Ther. 2016. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Lee, J.; Jung, E.; Kim, S.C.; Kang, J.I.; Lee, J.; Kim, Y.W.; Sung, Y.K.; Kang, H.K.; Park, D. A cell-based system for screening hair growth-promoting agents. Arch. Dermatol. Res. 2009, 301, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Oda, Y.; Matsuo, C.; Kumura, H.; Kobayashi, K. Stimulatory effect of Brazilian propolis on hair growth through proliferation of keratinocytes in mice. J. Agric. Food Chem. 2014, 62, 11854–11861. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.C.Q.; Taira, N.; Maruta, H.; Tawata, S. Artepillin C and other herbal PAK1-blockers: Effects on hair cell proliferation and related PAK1-dependent biological function in cell culture. Phytother. Res. 2016, 30, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, M.G.B.; Andrade, E.H.A.; Maia, J.G.S. Volatile constituents from leaves and flowers of Alpinia speciosa K. Schum. and A. purpurata (Viell.) Schum. Flavour Fragr. J. 1999, 14, 411–414. [Google Scholar] [CrossRef]
- Tawata, S.; Fukuta, M.; Xuan, T.D.; Deba, F. Total utilization of tropical plants Leucaena leucocephala and Alpinia zerumbet. J. Pestic. Sci. 2008, 33, 40–43. [Google Scholar] [CrossRef]
- Bezerra, M.A.C.; Leal-Cardoso, J.H.; Coelho-de-Souza, A.N.; Criddle, D.N.; Fonteles, M.C. Myorelaxant and antispasmodic effects of the essential oil of Alpinia speciosa on rat ileum. Phytother. Res. 2000, 14, 549–551. [Google Scholar] [CrossRef]
- Fujita, T.; Nishimura, H.; Kaburagi, K.; Mizutani, J. Plant growth inhibiting α-pyrones from Alpinia speciosa. Phytochemistry 1994, 36, 23–27. [Google Scholar] [CrossRef]
- Tawata, S.; Taira, S.; Kobamoto, N.; Ishihara, M.; Toyama, S. Syntheses and biological activities of dihydro-5,6-dehydrokawain derivatives. Biosci. Biotechnol. Biochem. 1996, 60, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Chompoo, J.; Kishimoto, W.; Makise, T.; Tawata, S. HIV-1 integrase and neuraminidase inhibitors from Alpinia zerumbet. J. Agric. Food Chem. 2011, 59, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Chompoo, J.; Upadhyay, A.; Fukuta, M.; Tawata, S. Effect of Alpinia zerumbet components on antioxidant and skin diseases-related enzymes. BMC Complement. Altern. Med. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chompoo, J.; Upadhyay, A.; Kishimoto, W.; Makise, T.; Tawata, S. Advanced glycation end products inhibitors from Alpinia zerumbet rhizomes. Food Chem. 2011, 129, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.C.Q.; Taira, N.; Tawata, S. Several herbal compounds in Okinawa plants directly inhibit the oncogenic/aging kinase PAK1. Drug Discov. Ther. 2014, 8, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mpalantinos, M.A.; Soares de Moura, R.; Parente, J.P.; Kuster, R.M. Biologically active flavonoids and kava pyrones from the aqueous extract of Alpinia zerumbet. Phytother. Res. 1998, 12, 442–444. [Google Scholar] [CrossRef]
- Shin, S.H.; Bak, S.S.; Kim, M.K.; Sung, Y.K.; Kim, J.C. Baicalin, a flavonoid, affects the activity of human dermal papilla cells and promotes anagen induction in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Wikramanayake, T.C.; Villasante, A.C.; Mauro, L.M.; Perez, C.I.; Schachner, L.A.; Jimenez, J.J. Prevention and treatment of alopecia areata with quercetin in the C3H/HeJ mouse model. Cell Stress Chaperones. 2012, 17, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Han, J.H.; Yoo, H.G.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine 2007, 14, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.S.; Park, S.J.; Hwang, S.L.; Lee, M.H.; Kim, C.D.; Lee, I.H.; Chang, S.Y.; Rang, M.J. The hair growth promoting effect of Asiasari radix extract and its molecular regulation. J. Dermatol. Sci. 2005, 38, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.S.; Thomas, L.N.; Douglas, R.C.; Lazier, C.B.; Rittmaster, R.S. Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J. Clin. Investig. 1996, 98, 2558–2563. [Google Scholar] [CrossRef] [PubMed]
- Shorter, K.; Farjo, N.P.; Picksley, S.M.; Randall, V.A. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 2008, 22, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates beta-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Lachgar, S.; Moukadiri, H.; Jonca, F.; Charveron, M.; Bouhaddioui, N.; Gall, Y.; Bonafe, J.L.; Plouet, J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J. Investig. Dermatol. 1996, 106, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Tiwari, R.; Srivastava, V.; Singh, T.B.; Asthana, R.K. Fresh water cyanobacteria Geitlerinema sp. CCC728 and Arthrospira sp. CCC729 as an anticancer drug resource. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rajalekshmi, D.S.; Kabeer, F.A.; Madhusoodhanan, A.R.; Bahulayan, A.K.; Prathapan, R.; Prakasan, N.; Varughese, S.; Nair, M.S. Anticancer activity studies of cubein isolated from Piper cubeba and its synthetic derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Be Tu, P.T.; Chompoo, J.; Tawata, S. Hispidin and related herbal compounds from Alpinia zerumbet inhibit both PAK1-dependent melanogenesis in melanocytes and reactive oxygen species (ROS) production in adipocytes. Drug Discov. Ther. 2015, 9, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tuo, W.; Hu, H.; Xu, J.; Chen, H.; Rao, Z.; Xiao, Y.; Hu, X.; Liu, P. Synthesis and activity evaluation of tilorone analogs as potential anticancer agents. Eur. J. Med. Chem. 2013, 64, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds kaempferol-3-O-β-d-glucuronide, labdadiene, MTD, TMOQ are available from the authors.



| Compound | IC50 (μM) |
|---|---|
| Labdadiene | 67.1 ± 6.0 b |
| MTD | 98.9 ± 9.3 a |
| TMOQ | 90.8 ± 1.6 a |
| KOG | 81.4 ± 1.9 a,b |
| Curcumin | 30.3 ± 2.9 c |
| Compound | IC50 (μM) |
|---|---|
| Labdadiene | 52.1 ± 3.0 a,b |
| MTD | 58.6 ± 2.5 a |
| TMOQ | 49.3 ± 0.7 b,c |
| KOG | 39.3 ± 2.4 c |
| Curcumin | 12.9 ± 1.1 d |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taira, N.; Nguyen, B.C.Q.; Tawata, S. Hair Growth Promoting and Anticancer Effects of p21-activated kinase 1 (PAK1) Inhibitors Isolated from Different Parts of Alpinia zerumbet. Molecules 2017, 22, 132. https://doi.org/10.3390/molecules22010132
Taira N, Nguyen BCQ, Tawata S. Hair Growth Promoting and Anticancer Effects of p21-activated kinase 1 (PAK1) Inhibitors Isolated from Different Parts of Alpinia zerumbet. Molecules. 2017; 22(1):132. https://doi.org/10.3390/molecules22010132
Chicago/Turabian StyleTaira, Nozomi, Binh Cao Quan Nguyen, and Shinkichi Tawata. 2017. "Hair Growth Promoting and Anticancer Effects of p21-activated kinase 1 (PAK1) Inhibitors Isolated from Different Parts of Alpinia zerumbet" Molecules 22, no. 1: 132. https://doi.org/10.3390/molecules22010132
APA StyleTaira, N., Nguyen, B. C. Q., & Tawata, S. (2017). Hair Growth Promoting and Anticancer Effects of p21-activated kinase 1 (PAK1) Inhibitors Isolated from Different Parts of Alpinia zerumbet. Molecules, 22(1), 132. https://doi.org/10.3390/molecules22010132