A Method for LC-MS/MS Profiling of Coumarins in Zanthoxylum zanthoxyloides (Lam.) B. Zepernich and Timler Extracts and Essential Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Targeted LC-MS2 Method
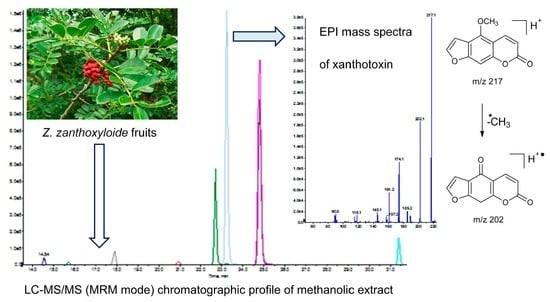
2.2. Analysis of Z. zanthoxyloides Extracts Using LC-MS2 Method
3. Materials and Methods
3.1. Solvents
3.2. Plant Material
3.3. Plant Extract and Essential Oil Preparation
3.4. References Compounds and Preparation of Standard Solutions
3.5. MS2 Conditions
3.6. LC Conditions
3.7. LC-MS2 Quantification
3.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miyake, Y.; Murakami, A.; Sugiyama, Y.; Isobe, M.; Koshimizu, K.; Ohigashi, H. Identification of coumarins from lemon fruit (Citrus limon) as inhibitors of in vitro tumor promotion and superoxide and nitric oxide generation. J. Agric. Food Chem. 1999, 47, 3151–3157. [Google Scholar] [CrossRef] [PubMed]
- Dugrand, A.; Olry, A.; Duval, T.; Hehn, A.; Froelicher, Y.; Bourgaud, F. Coumarin and Furanocoumarin Quantitation in Citrus Peel via Ultraperformance Liquid Chromatography Coupled with Mass Spectrometry (UPLC-MS). J. Agric. Food Chem. 2013, 61, 10677–10684. [Google Scholar] [CrossRef] [PubMed]
- Frérot, E.; Decorzant, E. Quantification of Total Furocoumarins in Citrus Oils by HPLC Coupled with UV, Fluorescence, and Mass Detection. J. Agric. Food Chem. 2004, 52, 6879–6886. [Google Scholar] [CrossRef] [PubMed]
- Peroutka, R.; Schulzová, V.; Botek, P.; Hajšlová, J. Analysis of furanocoumarins in vegetables (Apiaceae) and Citrus fruits (Rutaceae). J. Sci. Food Agric. 2007, 87, 2152–2163. [Google Scholar] [CrossRef]
- Rios, M.Y. Terpenes, Coumarins, and Flavones from Acacia pennatula. Chem. Nat. Compd. 2005, 41, 297–298. [Google Scholar] [CrossRef]
- Luo, K.; Sun, J.; Chan, J.Y.-W.; Yang, L.; Wu, S.; Fung, K.-P.; Liu, F. Anticancer effects of imperatorin isolated from Angelica dahurica: Induction of apoptosis in HepG2 cells through both death-receptor- and mitochondria-mediated pathways. Chemotherapy 2011, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Olomola, T.O.; Mosebi, S.; Klein, R.; Traut-Johnstone, T.; Coates, J.; Hewer, R.; Kaye, P.T. Novel furocoumarins as potential HIV-1 integrase inhibitors. Bioorg. Chem. 2014, 57, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Golfakhrabadi, F.; Abdollahi, M.; Ardakani, M.R.S.; Saeidnia, S.; Akbarzadeh, T.; Ahmadabadi, A.N.; Ebrahimi, A.; Yousefbeyk, F.; Hassanzadeh, A.; Khanavi, M. Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats. Pharm. Biol. 2014, 52, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, D.; Ashokkumar, N. Antihyperglycemic effect of esculetin modulated carbohydrate metabolic enzymes activities in streptozotocin induced diabetic rats. J. Funct. Foods 2012, 4, 776–783. [Google Scholar] [CrossRef]
- Schlatter, J.; Zimmerli, B.; Dick, R.; Panizzon, R.; Schlatter, C. Dietary intake and risk assessment of phototoxic furocoumarins in humans. Food Chem. Toxicol. 1991, 29, 523–530. [Google Scholar] [CrossRef]
- Belogurov, A.A.; Zavilgelsky, G.B. Mutagenic effect of furocoumarin monoadducts and cross-links on bacteriophage lambda. Mutat. Res. 1981, 84, 11–15. [Google Scholar] [CrossRef]
- Lake, B.G. Coumarin Metabolism, Toxicity and Carcinogenicity: Relevance for Human Risk Assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Lake, B.G.; Grasso, P. Comparison of the hepatotoxicity of coumarin in the rat, mouse, and Syrian hamster: A dose and time response study. Toxicol. Sci. 1996, 34, 105–117. [Google Scholar] [CrossRef]
- Guo, L.; Yamazoe, Y. Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines. Acta Pharmacol. Sin. 2004, 25, 129–136. [Google Scholar] [PubMed]
- European Union/European Council. Directive, 200315/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Off. J. Eur. Union 2003, 66, 26–35. [Google Scholar]
- Scientific Committee on Consumer Products (SCCP); Opinion on Furocoumarins in Cosmetic Products. SCCP/0942/05. 13 December 2005. Available online: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_036.pdf (accessed on 19 January 2017).
- Panel, E. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contacts with Food (AFC) on a request from the Commission related to Coumarin. EFSA J. 2004, 104, 1–36. [Google Scholar]
- Priess, H.W. Zur Kenntnis der Inhaltsstoffe von Fagara xanthoxyloides Lam. Berichte Dtsch. Pharm. Ges. 1911, 21, 227–267. [Google Scholar]
- Thoms, H. Über die Konstitution des Xanthotoxins und seine Beziehungen zum Bergapten. Berichte Dtsch. Chem. Ges. 1911, 44, 3325–3332. [Google Scholar] [CrossRef]
- Paris, R.; Moyse Mignon, H. Etude Préliminaire du Fagara Xanthoxyloides Lam. Ann. Pharm. Fr. 1947, 5, 410–420. [Google Scholar]
- Adesina, S.K. Further novel constituents of Zanthoxylum zanthoxyloides root and pericarp. J. Nat. Prod. 1986, 49, 715–716. [Google Scholar] [CrossRef]
- Tine, Y.; Diop, A.; Diatta, W.; Desjobert, J.-M.; Boye, C.S.B.; Wélé, A.; Costa, J.; Paolini, J. Chemical diversity and antimicrobial activity of volatile compounds from Zanthoxylum zanthoxyloides Lam. according to compound classes, plant organs and Senegalese sample locations. Chem. Biodivers. 2016. [Google Scholar] [CrossRef] [PubMed]
- Menut, C.; Lamaty, G.; Bessière, J.-M.; Molangui, T.; Ayedoun, M.A.; Sossou, P.V.; Sohounhloue, K.D.; Djossou, L.; Houenon, J.G. Aromatic plants of tropical West Africa. X. Volatile constituents of Zanthoxylum zanthoxyloides Lam. leaves and fruit pericarps from Benin. J. Essent. Oil Res. 2000, 12, 33–35. [Google Scholar] [CrossRef]
- Gardini, F.; Belletti, N.; Ndagijimana, M.; Guerzoni, M.E.; Tchoumbougnang, F.; Zollo, P.H.A.; Micci, C.; Lanciotti, R.; Kamdem, S.L.S. Composition of four essential oils obtained from plants from Cameroon, and their bactericidal and bacteriostatic activity against Listeria monocytogenes, Salmonella enteritidis and Staphylococcus aureus. Afr. J. Microbiol. Res. 2009, 3, 264–271. [Google Scholar]
- Dongmo, P.M.; Tchoumbougnang, F.; Sonwa, E.T.; Kenfack, S.M.; Zollo, P.H.; Menut, C. Antioxidant and anti-inflammatory potential of essential oils of some Zanthoxylum (Rutaceae) of Cameroon. Int. J. Essent. Oil Ther. 2008, 2, 82–88. [Google Scholar]
- Tatsadjieu, L.N.; Essia Ngang, J.J.; Ngassoum, M.B.; Etoa, F.-X. Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloides and Zanthoxylum leprieurii from Cameroon. Fitoterapia 2003, 74, 469–472. [Google Scholar] [CrossRef]
- Ngassoum, M.B.; Essia-Ngang, J.J.; Tatsadjieu, L.N.; Jirovetz, L.; Buchbauer, G.; Adjoudji, O. Antimicrobial study of essential oils of Ocimum gratissimum leaves and Zanthoxylum xanthoxyloides fruits from Cameroon. Fitoterapia 2003, 74, 284–287. [Google Scholar] [CrossRef]
- Fogang, H.P.D.; Tapondjou, L.A.; Womeni, H.M.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Lupidi, G.; Maggi, F.; Papa, F.; et al. Characterization and biological activity of essential oils from fruits of Zanthoxylum xanthoxyloides Lam. and Z. leprieurii Guill. & Perr., two culinary plants from Cameroon. Flavour Fragr. J. 2012, 27, 171–179. [Google Scholar]
- Adesina, S.K. The Nigerian Zanthoxylum; chemical and biological values. Afr. J. Tradit. Complement. Altern. Med. 2005, 2, 282–301. [Google Scholar] [CrossRef]
- Couillerot, E.; Caron, C.; Comoe, L.; Audran, J.-C.; Molinatti, P.; Zeches, M.; Le Men-Olivier, L.; Jardillier, J.-C.; Chenieux, J.-C. Benzophenanthridine and furoquinoline accumulation in cell suspension cultures of Fagara zanthoxyloides. Phytochemistry 1994, 37, 425–428. [Google Scholar] [CrossRef]
- Messmer, W.M.; Tin-wa, M.; Fong, H.H.S.; Bevelle, C.; Farnsworth, N.R.; Abraham, D.J.; Trojánek, J. Fagaronine, a New Tumor Inhibitor Isolated from Fagara zanthoxyloides Lam. (Rutaceae). J. Pharm. Sci. 1972, 61, 1858–1859. [Google Scholar] [CrossRef] [PubMed]
- Azando, E.V.B.; Hounzangbé-Adoté, M.S.; Olounladé, P.A.; Brunet, S.; Fabre, N.; Valentin, A.; Hoste, H. Involvement of tannins and flavonoids in the in vitro effects of Newbouldia laevis and Zanthoxylum zanthoxyloïdes extracts on the exsheathment of third-stage infective larvae of gastrointestinal nematodes. Vet. Parasitol. 2011, 180, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Bowden, K.; Ross, W.J. The local anæsthetic in Fagara xanthoxyloides. J. Chem. Soc. 1963, 3503–3505. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Sun, J.; Han, J.; Guo, D.-A. Chromatographic fingerprint analysis and characterization of furocoumarins in the roots of Angelica dahurica by HPLC/DAD/ESI-MSn technique. J. Pharm. Biomed. Anal. 2008, 47, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Prosen, H.; Kočar, D. Different sample preparation methods combined with LC–MS/MS and LC–UV for determination of some furocoumarin compounds in products containing citruses. Flavour Fragr. J. 2008, 23, 263–271. [Google Scholar] [CrossRef]
- Macmaster, A.P.; Owen, N.; Brussaux, S.; Brevard, H.; Hiserodt, R.; Leijs, H.; Bast, N.; Weber, B.; Loesing, G.; Sherlock, A.; et al. Quantification of selected furocoumarins by high-performance liquid chromatography and UV-detection: Capabilities and limits. J. Chromatogr. A 2012, 1257, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Park, A.Y.; Park, S.-Y.; Lee, J.; Jung, M.; Kim, J.; Kang, S.S.; Youm, J.-R.; Han, S.B. Simultaneous determination of five coumarins in Angelicae dahuricae Radix by HPLC/UV and LC-ESI-MS/MS. Biomed. Chromatogr. 2009, 23, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Mondello, L.; Dugo, L.; Stancanelli, R.; Dugo, G. LC-MS for the identification of oxygen heterocyclic compounds in Citrus essential oils. J. Pharm. Biomed. Anal. 2000, 24, 147–154. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wang, J.; Zhang, L.; Gao, B.; Shi, S.; Wang, X.; Li, J.; Tu, P. Simultaneous characterisation of fifty coumarins from the roots of Angelica dahurica by off-line two-dimensional high-performance liquid chromatography coupled with electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2014, 25, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Vallverdú-Queralt, A.; Negreira, N.; Simal-Gándara, J.; Lamuela-Raventós, R.M. Identification and quantification of grapefruit juice furanocoumarin metabolites in urine: An approach based on ultraperformance liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry and solid-phase extraction coupled to ultraperformance liquid chromatography coupled to triple quadrupole-tandem mass spectrometry. J. Agric. Food Chem. 2014, 62, 2134–2140. [Google Scholar] [PubMed]
- Wei, L.; Wang, X.; Zhang, P.; Sun, Y.; Jia, L.; Zhao, J.; Dong, S.; Sun, L. An UPLC-MS/MS method for simultaneous quantitation of two coumarins and two flavonoids in rat plasma and its application to a pharmacokinetic study of Wikstroemia indica extract. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1008, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.-H.; Zhang, Y.-B.; Yang, X.-W. Simultaneous determination and pharmacokinetics of sixteen Angelicae dahurica coumarins in vivo by LC–ESI-MS/MS following oral delivery in rats. Phytomedicine 2016, 23, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Messer, A.; Nieborowski, A.; Strasser, C.; Lohr, C.; Schrenk, D. Major furocoumarins in grapefruit juice I: Levels and urinary metabolite(s). Food Chem. Toxicol. 2011, 49, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Matu, E.N. Zanthoxylum zanthoxyloides (Lam.) Zepern. & Timler. Record from Protabase. Schmelzer, G.H., Gurib-Fakim, A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources Végétales de l’Afrique Tropicale): Wageningen, The Netherlands. Available online: http://www.prota4u.org/protav8.asp?p=Zanthoxylum+zanthoxyloides (accessed on 28 April 2016).
- Kerharo, J.; Adam, J.G. La Pharmacopée Sénégalaise Traditionnelle; Vigot Frères: Paris, France, 1974. [Google Scholar]
- Yang, W.; Ye, M.; Liu, M.; Kong, D.; Shi, R.; Shi, X.; Zhang, K.; Wang, Q.; Lantong, Z. A practical strategy for the characterization of coumarins in Radix glehniae by liquid chromatography coupled with triple quadrupole-linear ion trap mass spectrometry. J. Chromatogr. A 2010, 1217, 4587–4600. [Google Scholar] [CrossRef] [PubMed]
- Milesi, S.; Massot, B.; Gontier, E.; Bourgaud, F.; Guckert, A. Ruta graveolens L.: A promising species for the production of furanocoumarins. Plant Sci. 2001, 161, 189–199. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 3rd ed.; Council of Europe: Strasbourg, France, 1997. [Google Scholar]
- Bioanalytical Method Validation. Available online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf (accessed on 22 June 2016).
- Taylor, P.J. Matrix effects: The Achilles heel of quantitative high-performance liquid chromatography–electrospray–tandem mass spectrometry. Clin. Biochem. 2005, 38, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.




| Compounds | Tr (min) | Transition | MS Parameters (V) | |||||
|---|---|---|---|---|---|---|---|---|
| Q1 Mass (Da) | Q3 Mass (Da) | DP | EP | CEP | CE | CXP | ||
| 4-Methyldaphnetin | 9.5 | 193.1 | 119.2 | 56 | 8.0 | 12 | 31 | 4 |
| Esculetin | 11.7 | 179.0 | 123.1 | 71 | 9.0 | 10 | 27 | 4 |
| 6-Hydroxycoumarin | 13.6 | 163.0 | 107.2 | 51 | 5.5 | 16 | 31 | 4 |
| Isoscopoletin | 14.1 | 193.1 | 133.1 | 56 | 12.0 | 12 | 25 | 4 |
| 6,7-Dihydroxy-4-methylcoumarin | 14.3 | 193.1 | 91.2 | 76 | 10.5 | 10 | 37 | 4 |
| Daphnetin 7-methylether | 14.4 | 193.1 | 178.1 | 66 | 10.0 | 12 | 27 | 4 |
| Umbelliferone | 14.5 | 163.0 | 107.2 | 56 | 9.5 | 12 | 29 | 4 |
| Scopoletin | 14.5 | 193.1 | 133.2 | 76 | 8.5 | 10 | 25 | 4 |
| 5,7-Dihydroxy-4-methylcoumarin | 14.6 | 193.1 | 91.1 | 66 | 11.0 | 10 | 39 | 4 |
| 8-Acetyl-6-hydroxy-7-methoxycoumarin | 15.6 | 235.1 | 189.1 | 56 | 7.5 | 14 | 17 | 4 |
| Fraxidin | 15.7 | 223.1 | 190.1 | 61 | 9.5 | 14 | 19 | 4 |
| Xanthotoxol | 17.9 | 203.1 | 147.1 | 76 | 10.5 | 12 | 27 | 4 |
| 6,7-Dimethylesculetin | 17.9 | 207.1 | 151.2 | 61 | 4.5 | 12 | 29 | 4 |
| Coumarin | 18.9 | 147.0 | 91.1 | 46 | 4.5 | 12 | 29 | 4 |
| 8-Acetyl-7-methoxycoumarin | 18.9 | 219.1 | 115.2 | 61 | 8.5 | 12 | 43 | 4 |
| Herniarin | 20.8 | 177.1 | 121.1 | 56 | 4.0 | 12 | 27 | 4 |
| 4-Methoxycoumarin | 20.9 | 177.1 | 118.1 | 61 | 3.0 | 12 | 27 | 4 |
| 8-Acetyl-6,7-dimethoxycoumarin | 21.4 | 249.1 | 115.2 | 56 | 10.5 | 14 | 43 | 4 |
| 3-Acetylcoumarin | 21.6 | 189.1 | 115.1 | 41 | 8.5 | 12 | 37 | 4 |
| 7-Methylcoumarin | 22.7 | 161.1 | 105.1 | 51 | 8.5 | 12 | 29 | 4 |
| Psoralen | 22.7 | 187.1 | 131.1 | 56 | 10.5 | 12 | 33 | 4 |
| Nordalbergin | 22.7 | 255.1 | 152.2 | 76 | 10.0 | 14 | 55 | 4 |
| 6-Methoxy-4-methylcoumarin | 22.8 | 191.1 | 91.2 | 71 | 5.0 | 12 | 51 | 4 |
| 7-Methoxy-4-methylcoumarin | 23.2 | 191.1 | 91.2 | 71 | 4.0 | 12 | 53 | 4 |
| Xanthotoxin | 23.2 | 217.1 | 202.0 | 71 | 12.0 | 14 | 61 | 4 |
| 6-Methylcoumarin | 23.7 | 161.0 | 105.1 | 46 | 9.0 | 10 | 27 | 4 |
| Dalbergin | 24.2 | 269.1 | 152.2 | 91 | 10.5 | 16 | 59 | 4 |
| Citropten | 24.6 | 207.1 | 121.3 | 61 | 10.5 | 12 | 33 | 4 |
| Bergapten | 24.7 | 217.1 | 202.0 | 61 | 8.5 | 14 | 27 | 4 |
| Isopimpinllin | 24.9 | 247.1 | 217.1 | 71 | 10.5 | 14 | 23 | 4 |
| 7-Ethoxycoumarin | 25.0 | 191.1 | 163.1 | 56 | 4.5 | 12 | 25 | 4 |
| 4-Hydroxycoumarin | 25.1 | 163.0 | 121.1 | 81 | 9.0 | 10 | 25 | 4 |
| 4-Ethoxycoumarin | 25.2 | 191.1 | 163.0 | 46 | 9.0 | 12 | 21 | 4 |
| 4-Methylumbelliferone | 26.3 | 177.1 | 77.1 | 91 | 12.0 | 12 | 45 | 4 |
| 4-Methyl-7-ethoxycoumarin | 26.8 | 205.1 | 177.1 | 61 | 8.5 | 12 | 19 | 4 |
| Isobergapten | 28.7 | 217.1 | 202.1 | 61 | 8.5 | 12 | 33 | 4 |
| Bergaptol | 29.6 | 203.1 | 147.2 | 66 | 4.5 | 12 | 29 | 4 |
| Imperatorin | 31.5 | 271.2 | 203.1 | 51 | 5.0 | 14 | 17 | 4 |
| Osthol | 32.3 | 245.1 | 189.1 | 56 | 4.5 | 14 | 17 | 4 |
| No. | Compounds | Regression Equation | r2 | Linear Range (mg/L) | LOQ (mg/L) | LOD (mg/L) |
|---|---|---|---|---|---|---|
| 1 | Isoscopoletin | y = 112,000x + 3270 | 0.9970 | 0.10–2.5 | 0.10 | 0.03 |
| 2 | Daphnetin-7-methylether | y = 27,200x + 1260 | 0.9934 | 0.10–1.0 | 0.10 | 0.03 |
| 3 | Umbelliferone | y = 705,000x + 1580 | 0.9991 | 0.01–5.0 | 0.01 | 0.003 |
| 4 | Scopoletin | y = 119,000x + 2450 | 0.9961 | 0.10–5.0 | 0.10 | 0.03 |
| 5 | 6,7-Dimethylesculetin | y = 2,090,000x − 21,000 | 0.9974 | 0.10–5.0 | 0.10 | 0.03 |
| 6 | Coumarin | y = 241,000x − 2300 | 0.9979 | 0.01–5.0 | 0.01 | 0.003 |
| 7 | Herniarin | y = 4,080,000x − 3140 | 0.9989 | 0.01–5.0 | 0.01 | 0.003 |
| 8 | 4-Methoxycoumarin | y = 1,830,000x − 18,300 | 0.9941 | 0.01–5.0 | 0.01 | 0.003 |
| 9 | 7-Methylcoumarin | y = 1,170,000x − 2100 | 0.9973 | 0.10–5.0 | 0.10 | 0.03 |
| 10 | 6-Methylcoumarin | y = 874,000x − 11,500 | 0.9996 | 0.01–5.0 | 0.01 | 0.003 |
| 11 | Psoralen | y = 2,150,000x − 3000 | 0.9981 | 0.01–5.0 | 0.01 | 0.003 |
| 12 | Xanthotoxin | y = 1,180,000x + 472 | 0.9997 | 0.01–5.0 | 0.01 | 0.003 |
| 13 | Bergapten | y = 4,330,000x − 1850 | 0.9989 | 0.01–1.0 | 0.01 | 0.003 |
| 14 | Isopimpinellin | y = 3,330,000x + 721 | 0.9985 | 0.01–5.0 | 0.01 | 0.003 |
| 15 | Isobergapten | y = 2,690,000x − 1410 | 0.9976 | 0.01–5.0 | 0.01 | 0.003 |
| 16 | Imperatorin | y = 1,04,000x − 89 | 0.9951 | 0.01–5.0 | 0.01 | 0.003 |
| IS | 8-Acetyl-6-hydroxy-7-methoxycoumarin | y = 117,000x − 23.7 | 0.9992 | 0.01–2.5 | 0.01 | 0.003 |
| No. | Components | Concentration of Coumarin Components (mg/kg Dry Plant Material Weight ± SD) | Concentration of Coumarin Components (mg/kg Fruit Oil Weight ± SD) | |||||
|---|---|---|---|---|---|---|---|---|
| Solvent Extracts | Essential Oil | |||||||
| Fruit | Leaf | Root | Stem | Trunk | Fruit | |||
| 1 | Isoscopoletin | 632.5 ± 20.3 | <LOQ | <LOQ | 118.3 ± 3.8 | 1047.8 ± 18.4 | ND | ND |
| 2 | Daphnetin-7-methylether | 1116.0 ± 21.6 | ND | <LOQ | 99.4 ± 6.5 | 1835.9 ± 35.1 | <LOQ | <LOQ |
| 3 | Umbelliferone | 1243.1 ± 26.9 | ND | ND | ND | ND | ND | ND |
| 4 | Scopoletin | 370.6 ± 8.6 | <LOQ | <LOQ | <LOQ | 577.8 ± 3.1 | ND | ND |
| 5 | 6,7-Dimethylesculetin | 1074.3 ± 4.6 | <LOQ | <LOQ | <LOQ | 1062.0 ± 5.3 | <LOQ | <LOQ |
| 6 | Coumarin | ND | ND | ND | <LOQ | <LOQ | 0.1 ± 0.0 | 8.1 ± 0.6 |
| 7 | Herniarin | 152.0 ± 2.2 | ND | ND | ND | 79.3 ± 1.4 | 0.1 ± 0.0 | 6.5 ± 0.0 |
| 8 | 4-Methoxycoumarin | <LOQ | ND | ND | ND | <LOQ | tr | 2.0 ± 0.0 |
| 9 | 7-Methylcoumarin | <LOQ | ND | ND | ND | ND | tr | 3.4 ± 0.1 |
| 10 | 6-Methylcoumarin | <LOQ | ND | ND | ND | ND | tr | 4.4 ± 0.1 |
| 11 | Psoralen | 5192.6 ± 68.8 | 59.1 ± 2.3 | ND | <LOQ | ND | 2.3 ± 0.1 | 226.7 ± 6.2 |
| 12 | Xanthotoxin | 39,522.3 ± 9.3 | 263.7 ± 9.1 | <LOQ | 13.5 ± 0.5 | <LOQ | 4.2 ± 0.1 | 421.4 ± 12.5 |
| 13 | Bergapten | 8786.8 ± 29.8 | 84.7 ± 0.5 | ND | 6.9 ± 0.1 | ND | 3.00 ± 0.0 | 198.1 ± 2.0 |
| 14 | Isopimpinellin | 8439.3 ± 13.8 | 35.1 ± 1.4 | <LOQ | <LOQ | <LOQ | 0.4 ± 0.0 | 39.2 ± 0.0 |
| 15 | Isobergapten | 99.9 ± 1.4 | <LOQ | ND | ND | ND | tr | 1.5 ± 0.1 |
| 16 | Imperatorin | 29,607.0 ± 0.0 | 224.1 ± 9.9 | <LOQ | 16.5 ± 0.1 | <LOQ | 2.8 ± 0.1 | 284.4 ± 6.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tine, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. A Method for LC-MS/MS Profiling of Coumarins in Zanthoxylum zanthoxyloides (Lam.) B. Zepernich and Timler Extracts and Essential Oils. Molecules 2017, 22, 174. https://doi.org/10.3390/molecules22010174
Tine Y, Renucci F, Costa J, Wélé A, Paolini J. A Method for LC-MS/MS Profiling of Coumarins in Zanthoxylum zanthoxyloides (Lam.) B. Zepernich and Timler Extracts and Essential Oils. Molecules. 2017; 22(1):174. https://doi.org/10.3390/molecules22010174
Chicago/Turabian StyleTine, Yoro, Franck Renucci, Jean Costa, Alassane Wélé, and Julien Paolini. 2017. "A Method for LC-MS/MS Profiling of Coumarins in Zanthoxylum zanthoxyloides (Lam.) B. Zepernich and Timler Extracts and Essential Oils" Molecules 22, no. 1: 174. https://doi.org/10.3390/molecules22010174
APA StyleTine, Y., Renucci, F., Costa, J., Wélé, A., & Paolini, J. (2017). A Method for LC-MS/MS Profiling of Coumarins in Zanthoxylum zanthoxyloides (Lam.) B. Zepernich and Timler Extracts and Essential Oils. Molecules, 22(1), 174. https://doi.org/10.3390/molecules22010174