Comparison of Hydrogels Based on Commercial Chitosan and Beetosan® Containing Nanosilver
Abstract
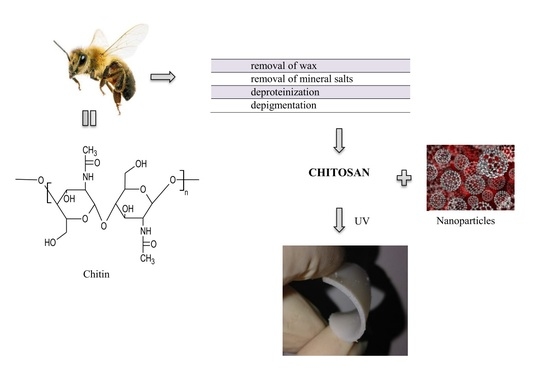
:1. Introduction
2. Results and Discussion
2.1. Swelling Studies
2.2. Incubation Studies
2.3. Results of FT-IR Spectroscopy
2.4. SEM Analysis of Hydrogels
2.5. Results of Studies on Cell Lines
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Beetosan®
3.2.2. Preparation of Silver Nanoparticles
3.2.3. Preparation of Hydrogels
3.2.4. Measurements of Sorption Capacity
3.2.5. Incubation Studies
3.2.6. FT-IR Spectroscopy
3.2.7. SEM Analysis
3.2.8. Studies on Cell Lines
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rajan, M.; Murugan, M.; Ponnamma, D.; Sadasivuni, K.K.; Munusamy, M.A. Poly-carboxylic acids functionalized chitosan nanocarriers for controlled and targeted anti-cancer drug delivery. Biomed. Pharmacother. 2016, 83, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Cerchiara, T.; Bigucci, F.; Gallucci, M.C.; Luppi, B. Mucoadhesive Buccal Tablets Based on Chitosan/Gelatin Microparticles for Delivery of Propranolol Hydrochloride. J. Pharm. Sci. 2015, 104, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Bigucci, F.; Abruzzo, A.; Saladini, B.; Gallucci, M.C.; Cerchiara, T.; Luppi, B. Development and characterization of chitosan/hyaluronan film for transdermal delivery of thiocolchicoside. Carbohydr. Polym. 2015, 130, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Abruzzo, A.; Parolin, C.; Vitali, B.; Bigucci, F.; Gallucci, M.C.; Nicoletta, F.P.; Luppi, B. Microparticles based on chitosan.carboxymethylcellulose polyelectrolyte complexes for colon delivery of vancomycin. Carbohydr. Polym. 2016, 143, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhou, W.; Wang, J.; Tang, R.; Zhang, D.; Wang, X. Hypromellose succinate-crosslinked chitosan hydrogel films for potential wound dressing. Int. J. Biol. Macromolec. 2016, 91, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yan, D.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Biomaterials based on N.N.N-trimethyl chitosan fibers in wound dressing applications. Int. J. Biol. Macromolec. 2016, 89, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, A.M.; Jancar, J.; Massoud, D.; Fohlerova, Z.; Elhadidy, H.; Spotz, Z.; Hebeish, A. Novel chitin/chitosan-glucan wound dressing: Isolation, characterization, antibacterial activity and wound healing properties. Int. J. Pharm. 2016, 510, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.M.S.; Schonherr, H. Enzyme-Sensing Chitosan Hydrogels. Langmuir 2014, 30, 7842–7850. [Google Scholar] [CrossRef] [PubMed]
- Nemtsev, S.V.; Zueva, O.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of Chitin and Chitosan from Honeybees. Appl. Biochem. Microbiol. 2004, 40, 39–43. [Google Scholar] [CrossRef]
- Draczyński, Z. Honeybee Corpses as an Available Source of Chitin. J. Appl. Polym. Sci. 2008, 109, 1974–1981. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Guo, Q.; Wang, Z.; Wang, H.; Yang, Y.; Huang, Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials 2012, 33, 6155–6161. [Google Scholar] [CrossRef] [PubMed]
- Tyliszczak, B.; Pielichowski, K. Novel hydrogels containing nanosilver for biomedical applications—Synthesis and characterization. J. Polym. Res. 2013, 20, 191–195. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, A.; Sharma, D.; Guatam, D.; Bhan, S.; Sharma, A.; Kapil, A.; Gupta, B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C 2016, 64, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Storm-Versloot, M.N.; Vos, C.G.; Ubbink, D.T.; Vermeulen, H. Topical silver for preventing wound infection. Cochrane Database Syst Rev. 2010, 17. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Tyliszczak, B.; Burgieł, Z.J.; Malina, D.; Bialik-Wąs, K.; Wzorek, Z. Nanocząstki srebra jako składniki preparatów agrochemicznych. Przem. Chem. 2014, 93, 1730–1733. [Google Scholar]
- Castro-Mayorga, J.L.; Fabra, M.J.; Lagaron, J.M. Stabilized nanosilver based antimicrobial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocompposites of interest in active food packaging. Innov. Food Sci. Emerg. 2016, 33, 524–533. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds presented in the article are available from the authors.










| Range of Vibrations (cm−1) | Functional Groups | Type of Vibrations |
|---|---|---|
| 3200–3600 | –NH | stretching |
| 3200–3500 | –OH | stretching |
| 2850–3000 | –CH | stretching |
| 1500–1650 | –NH | deformation |
| 1370–1390 | –CH | deformation |
| 1080–1360 | –CN | stretching |
| 1000–1300 | –CO | stretching |
| 600–700 | –CH | deformation |
| Tested cells | Day 3 | Day 5 | Day 7 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % Total * | Alive ** | Dead *** | % Total | Alive | Dead | % Total | Alive | Dead | |
| Control | 94 | 96 | 3 | 83 | 89 | 9 | 80 | 99 | 1 |
| Jurkat cells | 79 | 22 | 74 | 63 | 27 | 68 | 67 | 32 | 67 |
| WEHI cell lines | 45 | 78 | 22 | - | - | - | - | - | - |
| Gelatin 2% (mL) | Chitosan 3% (mL) | Beetosan® 3% (mL) | AgNPs (250 ppm) (mL) | Crosslinking Agent (mL) | Photoinitiator (mL) | |
|---|---|---|---|---|---|---|
| 1. | 20 | 30 | - | 1 | 8 | 0.25 |
| 2. | 20 | 30 | - | 3 | 8 | 0.25 |
| 3. | 20 | 30 | - | 5 | 8 | 0.25 |
| 4. | 20 | - | 30 | 1 | 8 | 0.25 |
| 5. | 20 | - | 30 | 3 | 8 | 0.25 |
| 6. | 20 | - | 30 | 5 | 8 | 0.25 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyliszczak, B.; Drabczyk, A.; Kudłacik, S. Comparison of Hydrogels Based on Commercial Chitosan and Beetosan® Containing Nanosilver. Molecules 2017, 22, 61. https://doi.org/10.3390/molecules22010061
Tyliszczak B, Drabczyk A, Kudłacik S. Comparison of Hydrogels Based on Commercial Chitosan and Beetosan® Containing Nanosilver. Molecules. 2017; 22(1):61. https://doi.org/10.3390/molecules22010061
Chicago/Turabian StyleTyliszczak, Bożena, Anna Drabczyk, and Sonia Kudłacik. 2017. "Comparison of Hydrogels Based on Commercial Chitosan and Beetosan® Containing Nanosilver" Molecules 22, no. 1: 61. https://doi.org/10.3390/molecules22010061
APA StyleTyliszczak, B., Drabczyk, A., & Kudłacik, S. (2017). Comparison of Hydrogels Based on Commercial Chitosan and Beetosan® Containing Nanosilver. Molecules, 22(1), 61. https://doi.org/10.3390/molecules22010061