Recent Advances in the Synthesis of Spiroheterocycles via N-Heterocyclic Carbene Organocatalysis
Abstract
:1. Introduction
2. NHC-Catalyzed Synthesis of Spiroheterocycles
2.1. Synthesis of Racemic Spiroheterocycles via NHC-Catalyzed Reactions
2.1.1. Catalysis Involving Nucleophilic Breslow Intermediates
2.1.2. Catalysis Involving Acylazolium Intermediates
2.2. Synthesis of Chiral Spiroheterocycles via NHC-Catalyzed Reactions
2.2.1. Catalysis Involving Chiral Nucleophilic Breslow Intermediates
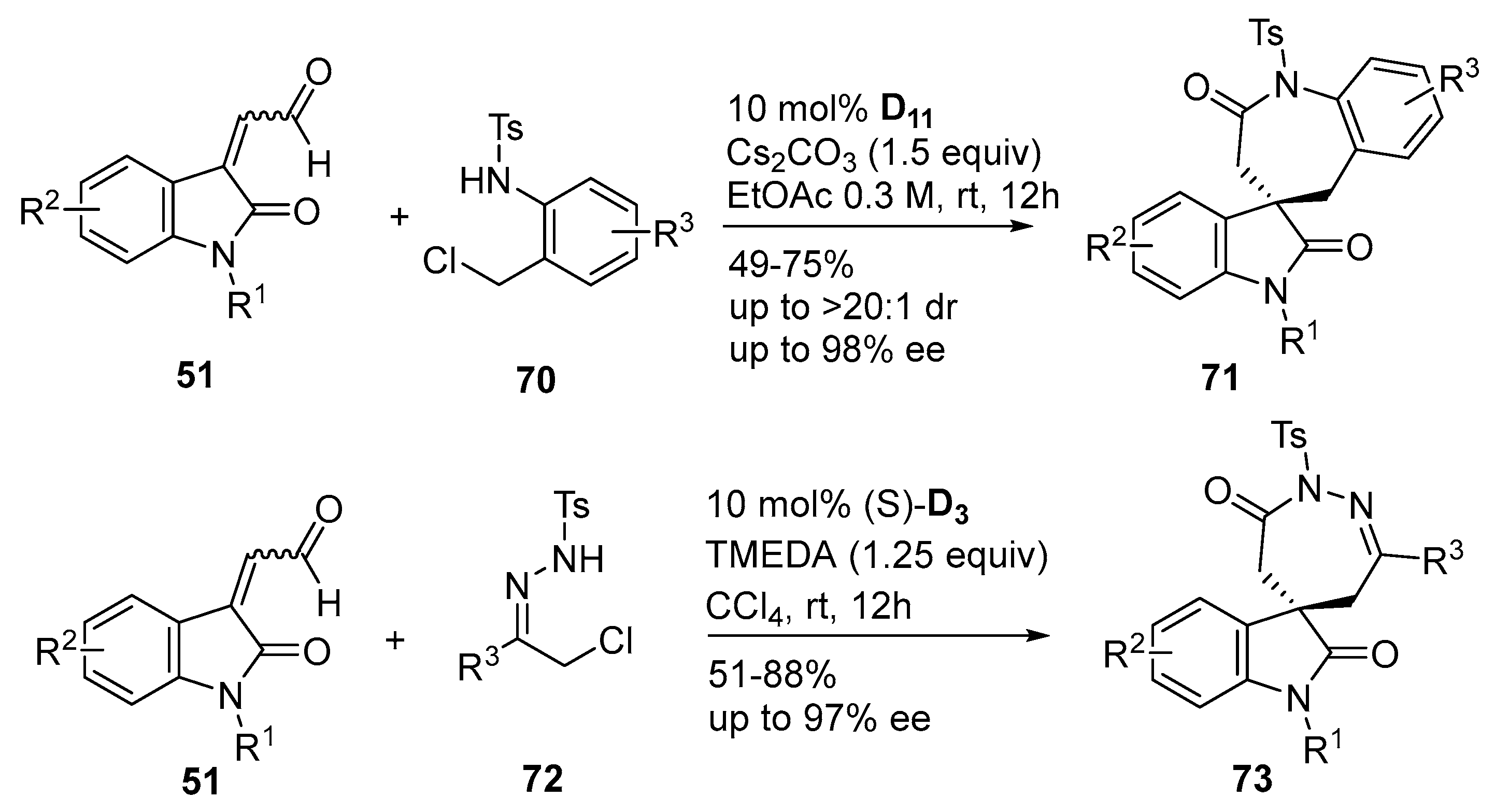
2.2.2. Catalysis Involving Chiral Acylazolium Intermediates
2.2.3. Catalysis Involving Radical Cation Intermediates
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. Engl. 2007, 46, 8748–8758. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Acharya, C.; Pal, U.; Chowdhury, S.R.; Sarkar, K.; Maiti, N.C.; Jaisankar, P.; Majumder, H.K. A novel spirooxindole derivative inhibits the growth of Leishmania donovani parasites both In Vitro and In Vivo by targeting type IB topoisomerase. Antimicrob. Agents Chemother. 2016, 60, 6281–6293. [Google Scholar] [CrossRef] [PubMed]
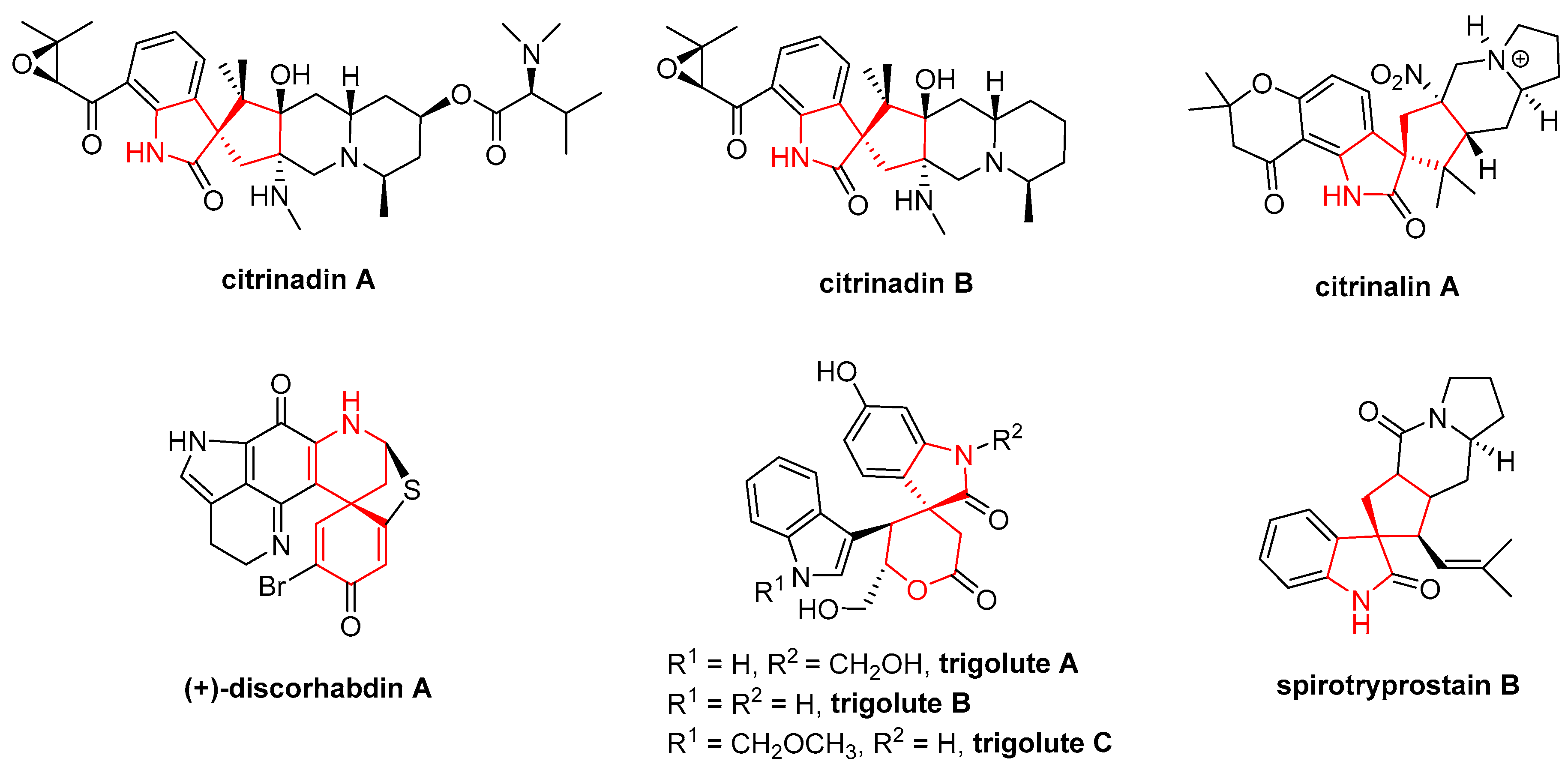
- Li, S.M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010, 27, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.M.; Perumal, S.; Yogeeswari, P.; Sriram, D. Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. Med. Chem. Commun. 2011, 2, 626–630. [Google Scholar] [CrossRef]
- Ding, K.; Lu, Y.; Nikolovska Coleska, Z.; Qiu, S.; Ding, Y.S.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P.P.; Tomita, Y. Structure-based design of potent non-peptide MDM2 inhibitors. J. Am. Chem. Soc. 2005, 127, 10130–10131. [Google Scholar] [CrossRef] [PubMed]
- Mugishima, T.; Tsuda, M.; Kasai, Y.; Ishiyama, H.; Fukushi, E.; Kawabata, J.; Watanabe, M.; Akao, K.; Kobayashi, J. Absolute stereochemistry of citrinadins A and B from marine-derived fungus. J. Org. Chem. 2005, 70, 9430–9435. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, E.F.; Vitamarques, A.M.; Tininis, A.; Seleghim, M.H.; Sette, L.D.; Veloso, K.; Ferreira, A.G.; Williams, D.E.; Patrick, B.O.; Dalisay, D.S. Use of experimental design for the optimization of the production of new secondary metabolites by two Penicillium species. J. Nat. Prod. 2010, 73, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Goey, A.K.; Chau, C.H.; Sissung, T.M.; Cook, K.M.; Venzon, D.J.; Castro, A.; Ransom, T.R.; Henrich, C.J.; McKee, T.C.; McMahon, J.B. Screening and biological effects of marine pyrroloiminoquinone alkaloids: Potential inhibitors of the HIF-1α/p300 interaction. J. Nat. Prod. 2016, 79, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.S.; Mei, W.L.; Guo, Z.K.; Liu, S.B.; Zhao, Y.X.; Yang, D.L.; Zeng, Y.B.; Jiang, B.; Dai, H.F. Two new types of bisindole alkaloid from Trigonostemon lutescens. Org. Lett. 2013, 15, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Li, J.L.; Yang, K.C.; Li, Y.; Li, Q.; Zhu, H.P.; Han, B.; Peng, C.; Zhi, Y.G.; Gou, X.J. Asymmetric synthesis of bicyclic dihydropyrans via organocatalytic inverse-electron-demand oxo-Diels-Alder reactions of enolizable aliphatic aldehydes. Chem. Commun. 2016, 52, 10617–10620. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Li, Q.; Yang, K.C.; Li, Y.; Zhou, L.; Han, B.; Peng, C.; Gou, X.J. A practical green chemistry approach to synthesize fused bicyclic 4H-pyranes via an amine catalysed 1,4-addition and cyclization cascade. RSC Adv. 2016, 6, 38875–38879. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, T.Y.; Chen, Y.C. Aminocatalytic asymmetric Diels–Alder reactions via HOMO activation. Acc. Chem. Res. 2012, 45, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Stambuli, J.P.; Silverman, S.M.; Schwörer, U. Palladium-catalyzed asymmetric [3+2] trimethylenemethane cycloaddition reactions. J. Am. Chem. Soc. 2006, 128, 13328–13329. [Google Scholar] [CrossRef] [PubMed]
- Posner, G.H.; Hamill, T.G. An asymmetric total synthesis of fragrant spiro[4.5]decane sesquiterpene (-)-β-vetivone via an enantiomerically pure vinylic sulfoxide. J. Org. Chem. 1988, 53, 6031–6035. [Google Scholar] [CrossRef]
- Yang, K.C.; Li, J.L.; Shen, X.D.; Li, Q.; Leng, H.J.; Huang, Q.; Zheng, P.K.; Gou, X.J.; Zhi, Y.G. Facile synthesis of novel spiroheterocycles via diastereoselective aziridination of cyclic enones. RSC Adv. 2017, 7, 21175–21179. [Google Scholar] [CrossRef]
- Dake, G.R.; Fenster, M.D.B.; Mélissa Fleury, A.; Patrick, B.O. Investigations of α-siloxy-epoxide ring expansions forming 1-azaspirocyclic ketones. J. Org. Chem. 2004, 69, 5676–5683. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, A.; Kumar, P.P. Claisen rearrangement based methodology for the spiroannulation of a cyclopentane ring. Formal total synthesis of (±)-acorone and isoacorones. Tetrahedron 2000, 56, 8189–8195. [Google Scholar] [CrossRef]
- Trost, B.M.; Cramer, N.; Silverman, S.M. Enantioselective construction of spirocyclic oxindolic cyclopentanes by palladium-catalyzed trimethylenemethane-[3+2]-cycloaddition. J. Am. Chem. Soc. 2007, 129, 12396–12397. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.Z.; Huang, L.; Liang, H.; Yang, K.C.; Leng, H.J.; Liu, Y.; Shen, X.D.; Gou, X.J.; Li, J.L. Diastereoselective synthesis of spirocyclopropanes under mild conditions via formal [2+1] cycloadditions using 2,3-dioxo-4-benzylidene-pyrrolidines. Molecules 2017, 22, 328. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhou, Y.; Chen, X.; Zhang, P.; Xu, J.; Liu, H. N-heterocyclic carbene catalytic [4+2] cyclization of 3-alkylenyloxindoles with enals: A gamma-carbon activation for enantioselective assembly of spirocarbocyclic oxindoles. J. Org. Chem. 2016, 81, 8888–8899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Li, N.K.; Yin, S.J.; Sun, B.B.; Fan, W.T.; Wang, X.W. Chiral N-heterocyclic carbene-catalyzed asymmetric Michael–intramolecular aldol-lactonization cascade for enantioselective construction of β-propiolactone-fused spiro[cyclopentane-oxindoles]. Adv. Synth. Catal. 2017, 359, 1541–1551. [Google Scholar] [CrossRef]
- Rong, X.; Yao, H.; Xia, W.; Du, Y.; Zhou, Y.; Liu, H. Enantioselective assembly of spirolactones through NHC-catalyzed remote γ-carbon addition of enals with isatins. ACS Comb. Sci. 2016, 18, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.K.; Hanhan, N.V.; Ball-Jones, N.R. Asymmetric catalysis for the synthesis of spirocyclic compounds. ACS Catal. 2013, 3, 540–553. [Google Scholar] [CrossRef]
- Turner, N.J. Applications of transketolases in organic synthesis. Curr. Opin. Biotechnol. 2000, 11, 527–531. [Google Scholar] [CrossRef]
- Stryer, L. Biochemistry, 4th ed.; Freedman and Company: New York, NY, USA, 1995; pp. 739–740. ISBN 978-0716720096. [Google Scholar]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef] [PubMed]
- Mizuhara, S.; Handler, P. Mechanism of thiamine-catalyzed reactions 1. J. Am. Chem. Soc. 1954, 76, 571–573. [Google Scholar] [CrossRef]
- Maki, B.E.; Chan, A.; Phillips, E.M.; Scheidt, K.A. N-heterocyclic carbene-catalyzed oxidations. Tetrahedron 2009, 65, 3102–3109. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.M.; Chan, A.; Scheidt, K.A. Discovering new reactions with N-heterocyclic carbene catalysis. Aldrichim. Acta 2009, 42, 55–66. [Google Scholar]
- Buchner, E.; Curtius, T. Ueber die einwirkung von diazoessigäther auf aromatische kohlenwasserstoffe. Eur. J. Inorg. Chem. 1885, 18, 2377–2379. [Google Scholar] [CrossRef]
- Staudinger, H.; Kupfer, O. Über reaktionen des methylens. III. Diazomethan. Eur. J. Inorg. Chem. 1912, 45, 501–509. [Google Scholar] [CrossRef]
- Igau, A.; Grutzmacher, H.; Baceiredo, A.; Bertrand, G. Analogous .alpha.,.alpha.′-bis-carbenoid, triply bonded species: Synthesis of a stable. lambda.3-phosphino carbene-.lambda. 5-phosphaacetylene. J. Am. Chem. Soc. 1988, 110, 6463–6466. [Google Scholar] [CrossRef]
- Igau, A.; Baceiredo, A.; Trinquier, G.; Bertrand, G. [Bis(diisopropylamino)phosphino] trimethylsilylcarbene: A stable nucleophilic carbene. Angew. Chem. Int. Ed. Engl. 1989, 28, 621–622. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
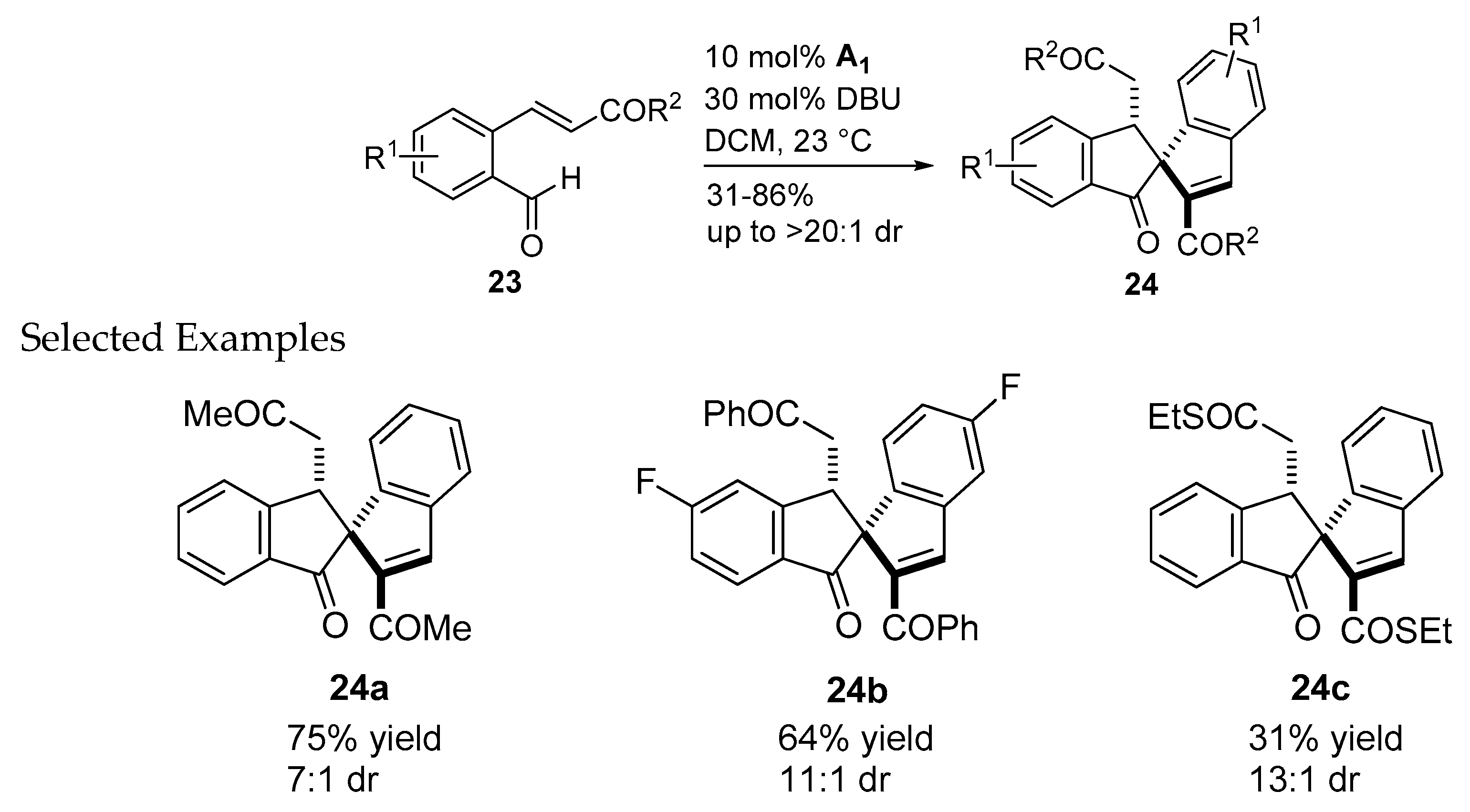
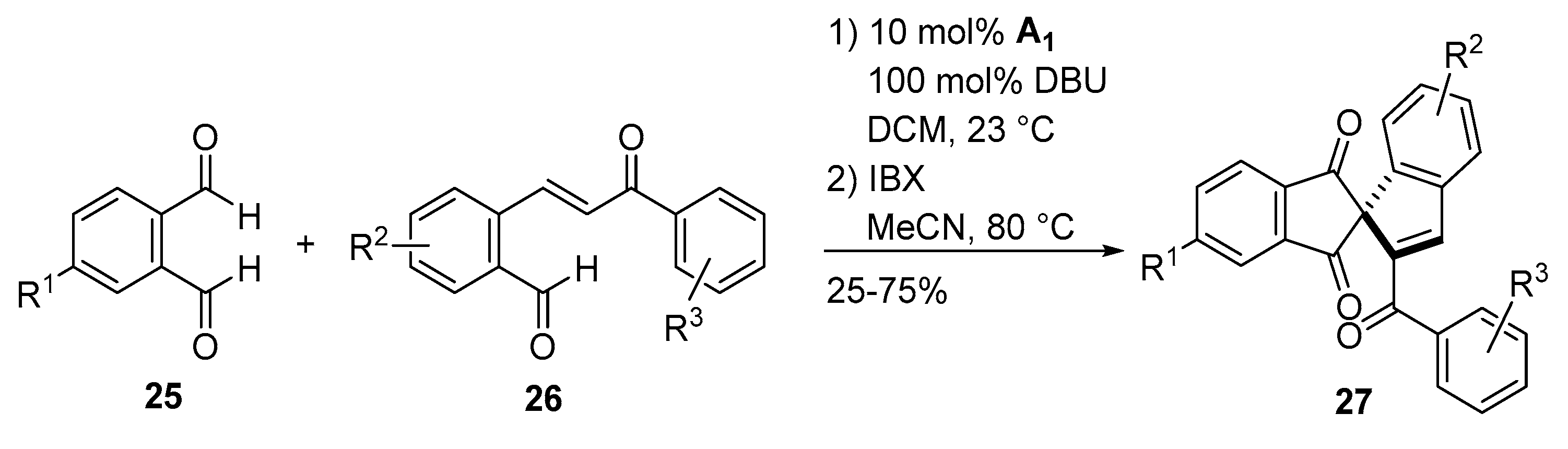
- Guo, C.; Schedler, M.; Daniliuc, C.G.; Glorius, F. N-Heterocyclic carbene catalyzed formal [3+2] annulation reaction of enals: An efficient enantioselective access to spiro-heterocycles. Angew. Chem. Int. Ed. Engl. 2014, 53, 10232–10236. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Balensiefer, T. Nucleophilic carbenes in asymmetric organocatalysis. Acc. Chem. Res. 2004, 37, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.H.; Shen, L.T.; Ye, S. Highly diastereo-and enantioselective NHC-catalyzed [3+2] annulation of enals and isatins. Chem. Commun. 2011, 47, 10136–10138. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Fu, L.; Wu, J.; Yang, K.C.; Li, Q.Z.; Gou, X.J.; Peng, C.; Han, B.; Shen, X.D. Highly enantioselective synthesis of fused bicyclic dihydropyranones via low-loading N-heterocyclic carbene organocatalysis. Chem. Commun. 2017, 53, 6875–6878. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Romanovmichailidis, F.; White, N.A.; Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R. On the mechanism of thiamine action. IV. 1 Evidence from studies on model systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Nair, V.; Vellalath, S.; Poonoth, M.; Mohan, R.; Suresh, E. N-heterocyclic carbene catalyzed reaction of enals and 1,2-dicarbonyl compounds: Stereoselective synthesis of spiro γ-butyrolactones. Org. Lett. 2006, 8, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Larios, E.; Holmes, J.M.; Daschner, C.L.; Gravel, M. NHC-catalyzed spiro bis-indane formation via domino Stetter-aldol-Michael and Stetter-aldol-aldol reactions. Org. Lett. 2011, 42, 5772–5775. [Google Scholar] [CrossRef]
- Sánchez-Larios, E.; Holmes, J.M.; Daschner, C.L.; Gravel, M. Synthesis of spiro bis-indanes via domino Stetter-aldol-Michael and Stetter-aldol-aldol reactions: Scope and limitations. Synthesis 2011, 2011, 1896–1904. [Google Scholar] [CrossRef]
- Jiang, K.; Tiwari, B.; Chi, Y.R. Access to spirocyclic oxindoles via N-heterocyclic carbene-catalyzed reactions of enals and oxindole-derived α,β-unsaturated imines. Org. Lett. 2012, 14, 2382–2385. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.S.; Xiao, Z.X.; Liu, R.; Li, T.J.; Jiao, W.H.; Yu, C.X. N-heterocyclic-carbene-catalyzed reaction of α-bromo-α,β-Unsaturated aldehyde or α,β-dibromoaldehyde with isatins: An efficient synthesis of spirocyclic oxindole–dihydropyranones. Chemistry 2013, 19, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Sahoo, B.; Daniliuc, C.G.; Glorius, F. Conjugate umpolung of β,β-disubstituted enals by dual catalysis with an N-heterocyclic carbene and a Bronsted acid: Facile construction of contiguous quaternary stereocenters. Angew. Chem. Int. Ed. Engl. 2014, 53, 10515–10519. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Hu, Z.; Jin, J.; Lu, Y.; Tang, W.; Wang, B.; Lu, T. N-heterocyclic carbene-catalyzed three-component domino reaction of alkynyl aldehydes with oxindoles. Org. Lett. 2012, 14, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.G.; Xu, J.Y.; Cao, J.; Fang, C.; Zhu, J.D.; Lu, T.; Du, D. N-heterocyclic carbene-mediated formal [3+3] annulation of isatin-derived α,β-unsaturated acids: Access to functionalized 3,4′-spirooxindole d-lactones. Tetrahedron 2017, 73, 3249–3254. [Google Scholar] [CrossRef]
- Zhao, L.L.; Li, X.S.; Cao, L.L.; Zhang, R.; Shi, X.Q.; Qi, J. Access to dihydropyridinones and spirooxindoles: Application of N-heterocyclic carbene-catalyzed [3+3] annulation of enals and oxindole-derived enals with 2-aminoacrylates. Chem. Commun. 2017, 53, 5985–5988. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Zhang, Y.Y.; Ye, S. Enantioselective synthesis of spirocyclic oxindole-β-lactones via N-heterocyclic carbene-catalyzed cycloaddition of ketenes and isatins. Adv. Synth. Catal. 2010, 352, 1892–1895. [Google Scholar] [CrossRef]
- Zhang, H.M.; Gao, Z.H.; Ye, S. Bifunctional N-heterocyclic carbene-catalyzed highly enantioselective synthesis of spirocyclic oxindolo-β-lactams. Org. Lett. 2014, 16, 3079–3081. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yuan, S.; Peng, J.; Miao, M.; Chen, Z.; Ren, H. Enantioselective [2+2] annulation of simple aldehydes with isatin-derived ketimines via oxidative N-heterocyclic carbene catalysis. Chem. Commun. 2017, 53, 3430–3433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.G.; Yao, W.J.; Zhang, Y.C.; Ma, C. Chiral spirooxindole-butenolide synthesis through asymmetric N-heterocyclic carbene-catalyzed formal (3+2) annulation of 3-bromoenals and isatins. Org. Lett. 2014, 16, 5028–5031. [Google Scholar] [CrossRef] [PubMed]
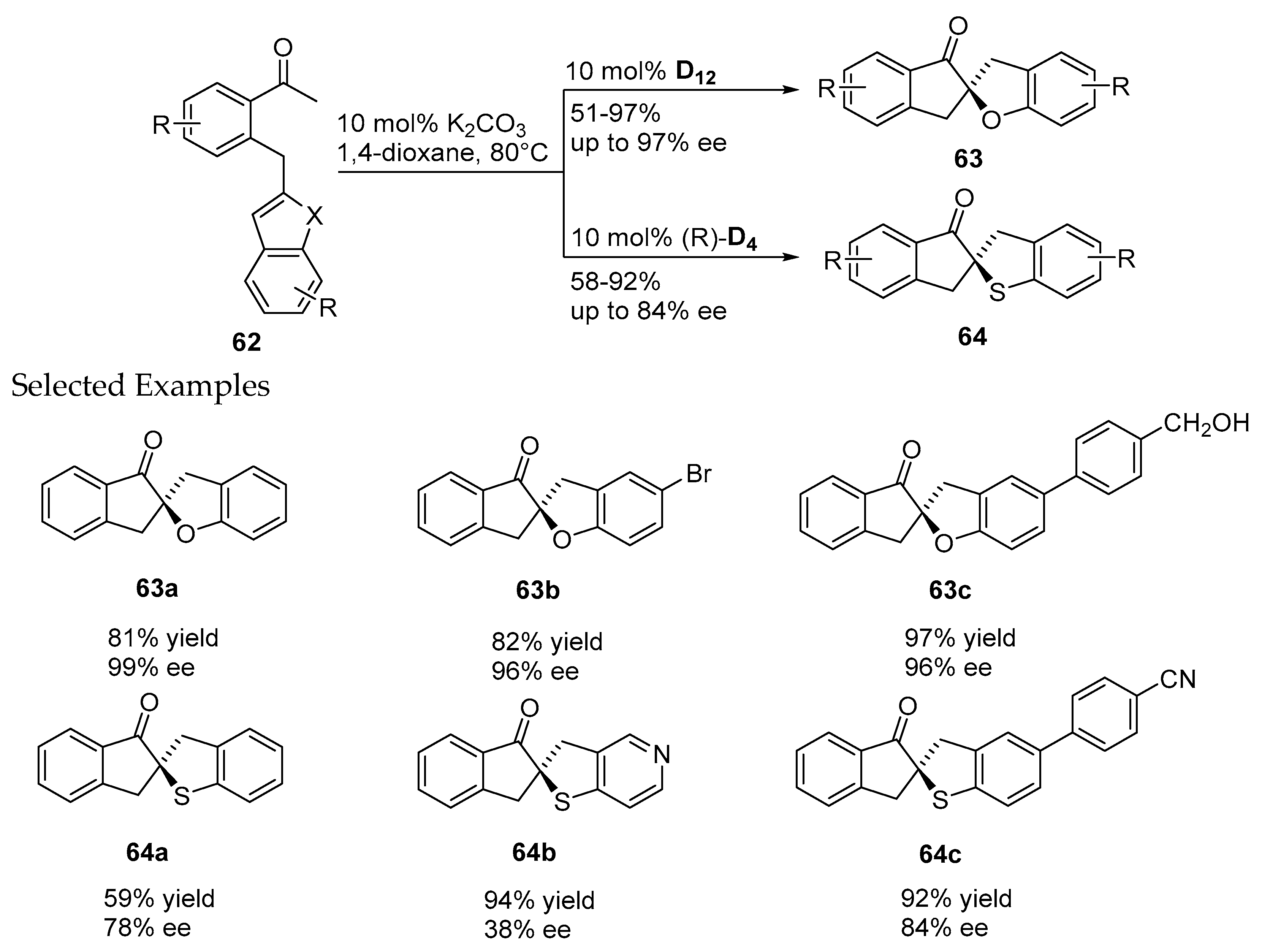
- Janssen-Müller, D.; Fleige, M.; Schluens, D.; Wollenburg, M.; Daniliuc, C.G.; Neugebauer, J.; Glorius, F. NHC-catalyzed enantioselective dearomatizing hydroacylation of benzofurans and benzothiophenes for the synthesis of spirocycles. ACS Catal. 2016, 6, 5735–5739. [Google Scholar] [CrossRef]
- Dugal-Tessier, J.; O’Bryan, E.A.; Schroeder, T.B.; Cohen, D.T.; Scheidt, K.A. An N-heterocyclic carbene/Lewis acid strategy for the stereoselective synthesis of spirooxindole lactones. Angew. Chem. Int. Ed. Engl. 2012, 51, 4963–4967. [Google Scholar] [CrossRef] [PubMed]
- Reddi, Y.; Sunoj, R.B. Origin of stereoselectivity in cooperative asymmetric catalysis involving N-heterocylic carbenes and Lewis acids toward the synthesis of spirooxindole lactone. ACS Catal. 2017, 7, 530–537. [Google Scholar] [CrossRef]
- Xu, J.F.; Yuan, S.R.; Miao, M.Z.; Chen, Z.K. 1-Hydroxybenzotriazole-assisted, N-heterocyclic carbene catalyzed β-functionalization of saturated carboxylic esters: Access to spirooxindole lactones. J. Org. Chem. 2016, 81, 11454–11460. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.F.; Yuan, S.R.; Miao, M.Z. N-Heterocyclic carbene catalyzed [4+2] annulation reactions with In Situ generated heterocyclic ortho-quinodimethanes. Org. Lett. 2016, 18, 3822–3825. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Blümel, M.; Philipps, A.R.; Wang, A.; Puttreddy, R.; Rissanen, K.; Enders, D. Asymmetric synthesis of spirobenzazepinones with atroposelectivity and spiro-1,2-diazepinones by NHC-catalyzed [3+4] annulation reactions. Angew. Chem. Int. Ed. Engl. 2016, 55, 11110–11114. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yu, C.; Li, T.; Wang, X.S.; Yao, C. N-heterocyclic carbene/Lewis acid strategy for the stereoselective synthesis of spirocyclic oxindole–dihydropyranones. Org. Lett. 2014, 16, 3632–3635. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.L.; Dong, S.D.; Tang, W.F.; Lu, T.; Du, D. N-heterocyclic carbene-catalyzed formal [3+2] annulation of α-bromoenals with 3-aminooxindoles: A stereoselective synthesis of spirooxindole γ-butyrolactams. J. Org. Chem. 2015, 80, 11593–11597. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Joseph, S.; Bhunia, A.; Gonnade, R.G.; Yetra, S.R.; Biju, A.T. Enantioselective synthesis of spiro γ-butyrolactones by N-heterocyclic carbene (NHC)-catalyzed formal [3+2] annulation of enals with 3-hydroxy oxindoles. Org. Biomol. Chem. 2017, 15, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Chen, K.Q.; Zhang, C.L.; Ye, S. N-heterocyclic carbene-catalyzed synthesis of spirocyclopentene-oxindoles from bromoenals. Chem. Commun. 2017, 53, 4327–4330. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Daniliuc, C.G.; Studer, A. Oxidative N-heterocyclic carbene catalyzed dearomatization of indoles to spirocyclic indolenines with a quaternary carbon stereocenter. Angew. Chem. Int. Ed. Engl. 2017, 56, 7402–7406. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Chen, K.Q.; Sun, D.Q.; Ye, S. N-heterocyclic carbene-catalyzed oxidative [3+2] annulation of dioxindoles and enals: Cross coupling of homoenolate and enolate. Chem. Sci. 2017, 8, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, L.; Wang, J.; Huang, B.; Zeng, K.; Jin, H.; Zhang, Q.; Jia, Y. Total synthesis of natural spiro-trisindole enantiomers similisines A, B and their stereoisomers. Tetrahedron Lett. 2017, 58, 1934–1938. [Google Scholar] [CrossRef]
- War May Zin, W.; Prompanya, C.; Buttachon, S.; Kijjoa, A. Bioactive secondary metabolites from a Thai collection of soil and marine-derived fungi of the genera Neosartorya and Aspergillus. Curr. Drug Deliv. 2016, 13, 378–388. [Google Scholar] [CrossRef]



























© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, X.; Zeng, R.; Zhang, Y.; Dai, Q.-S.; Leng, H.-J.; Gou, X.-J.; Li, J.-L. Recent Advances in the Synthesis of Spiroheterocycles via N-Heterocyclic Carbene Organocatalysis. Molecules 2017, 22, 1882. https://doi.org/10.3390/molecules22111882
Liu Y, Zhang X, Zeng R, Zhang Y, Dai Q-S, Leng H-J, Gou X-J, Li J-L. Recent Advances in the Synthesis of Spiroheterocycles via N-Heterocyclic Carbene Organocatalysis. Molecules. 2017; 22(11):1882. https://doi.org/10.3390/molecules22111882
Chicago/Turabian StyleLiu, Yue, Xiang Zhang, Rong Zeng, Ying Zhang, Qing-Song Dai, Hai-Jun Leng, Xiao-Jun Gou, and Jun-Long Li. 2017. "Recent Advances in the Synthesis of Spiroheterocycles via N-Heterocyclic Carbene Organocatalysis" Molecules 22, no. 11: 1882. https://doi.org/10.3390/molecules22111882
APA StyleLiu, Y., Zhang, X., Zeng, R., Zhang, Y., Dai, Q.-S., Leng, H.-J., Gou, X.-J., & Li, J.-L. (2017). Recent Advances in the Synthesis of Spiroheterocycles via N-Heterocyclic Carbene Organocatalysis. Molecules, 22(11), 1882. https://doi.org/10.3390/molecules22111882




