Design, Synthesis and Bioactivities of Novel Isoxazole-Containing Pyrazole Oxime Derivatives
Abstract
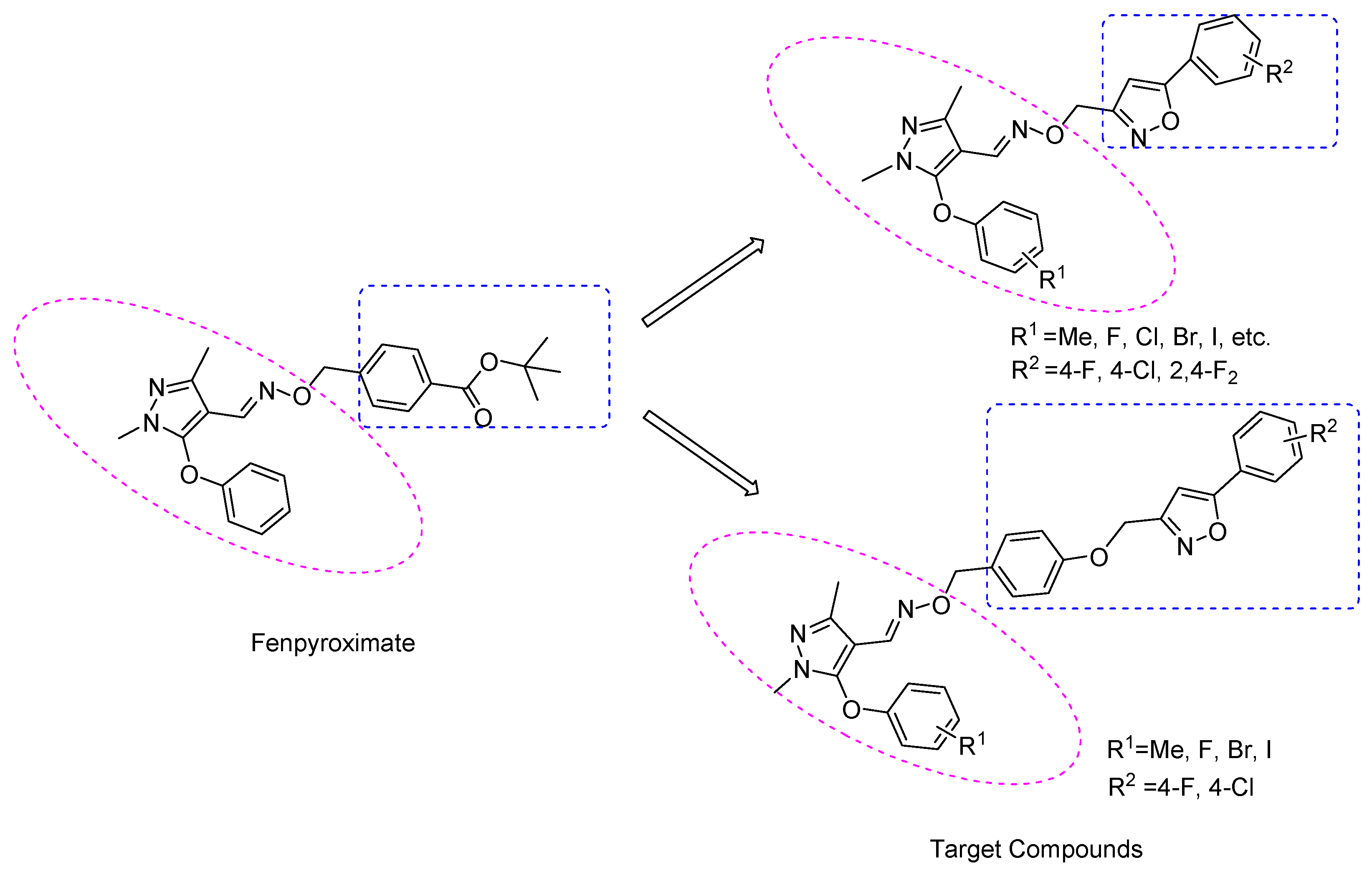
1. Introduction
2. Results and Discussion
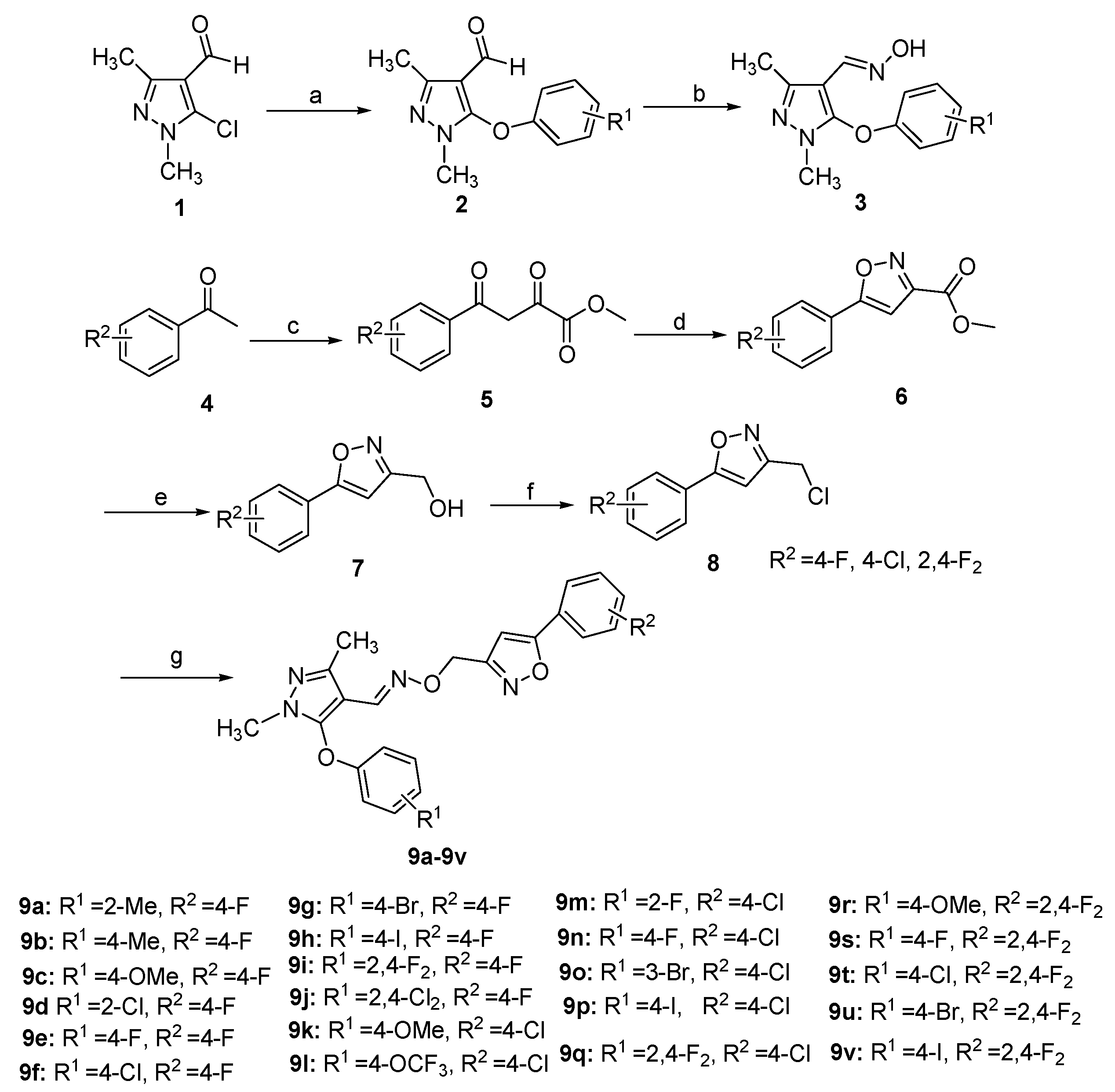
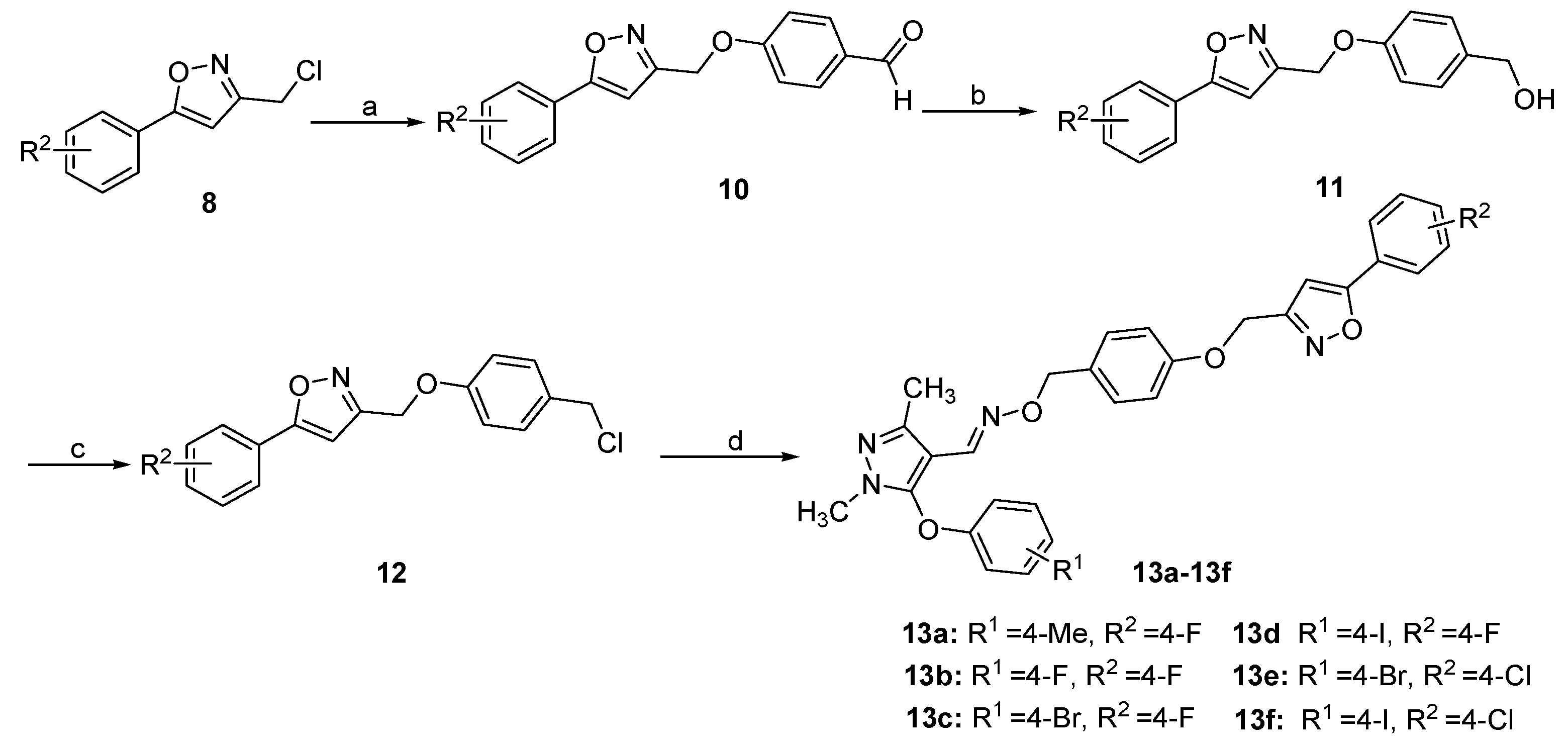
2.1. Chemistry
2.2. Biological Activities
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedures
3.1.2. General Procedure for the Preparation of 2
3.1.3. General Procedure for the Preparation of 3
3.1.4. General Procedure for the Preparation of 7
3.1.5. General Procedure for the Preparation of 8
3.1.6. General Procedure for the Preparation of 9a–9v
3.1.7. General Procedure for the Preparation of 10
3.1.8. General Procedure for the Preparation of 11
3.1.9. General Procedure for the Preparation of 12
3.1.10. General Procedure for the Preparation of 13a–13f
3.2. Biological Tests
3.2.1. Bioassay Methods
3.2.2. Insecticidal Activities against Mythimna separata
3.2.3. Acaricidal Activities against Tetranychus cinnabarinus, and Insecticidal Activities against Aphis medicaginis and Nilaparvata lugens
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, M.; Liu, G.; Zhang, Y.; Feng, T.; Xu, M.; Xu, H. Design, synthesis and evaluation of novel isoxazolines/oxime sulfonates of 2(2,6)-(di)chloropodophyllotoxins as insecticidal agents. Sci. Rep. 2016, 6, 33062. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ozoe, F.; Furuta, K.; Ozoe, Y. 4,5-Substituted 3-isoxazolols with insecticidal activity act as competitive antagonists of housefly GABA receptors. J. Agric. Food Chem. 2015, 63, 6304–6312. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wagerle, T.; Long, J.K.; Lahm, G.P.; Barry, J.D.; Smith, R.M. Insecticidal quinoline and isoquinoline isoxazolines. Bioorg. Med. Chem. Lett. 2014, 24, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Z.; Bin, L.; Cai, B.; Li, Y.; Wang, Q. Design, synthesis, and herbicidal activities of novel 2-cyanoacrylates containing isoxazole moieties. J. Agric. Food Chem. 2010, 58, 2685–2689. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.T.; Kim, H.R.; Jeon, D.J.; Hong, K.S.; Song, J.H.; Cho, K.Y. 5-(2,6-Difluorobenzyl) oxymethyl-5-methyl-3-(3-methylthiophen-2-yl)-1,2-isoxazoline as a useful rice herbicide. J. Agric. Food Chem. 2005, 53, 8639–8643. [Google Scholar] [CrossRef] [PubMed]
- Hamper, B.C.; Leschinsky, K.L.; Massey, S.S.; Bell, C.L.; Brannigan, L.H.; Prosch, S.D. Synthesis and herbicidal activity of 3-aryl-5-(haloalkyl)-4-isoxazolecarboxamides and their derivatives. J. Agric. Food Chem. 1995, 43, 219–228. [Google Scholar] [CrossRef]
- Liu, P.; Xu, Y.; Li, J.; Liu, J.; Cao, Y.; Liu, X. Photodegradation of the isoxazolidine fungicide SYP-Z048 in aqueous solution: Kinetics and photoproducts. J. Agric. Food Chem. 2012, 60, 11657–11663. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, D.G.; Andrei, G.; Schols, D.; Snoeck, R.; Grabkowska-Druzyc, M. New isoxazolidine-conjugates of quinazolinones-synthesis, antiviral and cytostatic activity. Molecules 2016, 21, 959. [Google Scholar] [CrossRef] [PubMed]
- Kokosza, K.; Andrei, G.; Schols, D.; Snoeck, R.; Piotrowska, D.G. Design, antiviral and cytostatic properties of isoxazolidine-containing amonafide analogues. Bioorg. Med. Chem. 2015, 23, 3135–3146. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, D.G.; Balzarini, J.; Glowacka, I.E. Design, synthesis, antiviral and cytostatic evaluation of novel isoxazolidine nucleotide analogues with a 1,2,3-triazole linker. Eur. J. Med. Chem. 2012, 47, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.N.; Dev, G.J.; Ravikumar, N.; Swaroop, D.K.; Debanjan, B.; Bharath, G.; Rao, A.G. Synthesis of novel triazole/isoxazole functionalized 7-(trifluoromethyl) pyrido[2,3-d]pyrimidine derivatives as promising anticancer and antibacterial agents. Bioorg. Med. Chem. Lett. 2016, 26, 2927–2930. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.J.; Iwamoto, S.; Robins, L.I.; Fettinger, J.C.; Sparks, T.C.; Lorsbach, B.A.; Kurth, M.J. 3-(Arylthiomethyl) isoxazole-4,5-dicarboxamides: Chemoselective nucleophilic chemistry and insecticidal activity. J. Agric. Food Chem. 2009, 57, 7422–7426. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, Y.; Xiong, L.; Liu, Y.; Wang, Q. Design, synthesis, and insecticidal evaluation of new benzoylureas containing isoxazoline and isoxazole group. J. Agric. Food Chem. 2011, 59, 4851–4859. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Pei, J.; Ning, Y.; Liu, M.; Shan, P.; Liu, J.; Zou, X. Synthesis and insecticidal activities of novel pyrazole oxime ether derivatives with different substituted pyridyl rings. Pest Manag. Sci. 2014, 70, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, Y.; Xiong, L.; Li, Y.; Yang, N.; Wang, Q. Design, synthesis, and insecticidal evaluation of new pyrazole derivatives containing imine, oxime ether, oxime ester, and dihydroisoxazoline groups based on the inhibitor binding pocket of respiratory complex I. J. Agric. Food Chem. 2013, 61, 8730–8736. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, D.; Fu, C.; Zou, X. Synthesis and biological activity evaluation of novel pyrazole oxime ether derivatives containing chlorothiazole group and pyrimidine rings. Chin. J. Org. Chem. 2015, 35, 100–108. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.Q.; Liu, J.; Yang, X.P.; Liu, Z.J. Stereoselective synthesis and antifungal activities of (E)-α-(methoxyimino) benzeneacetate derivatives containing 1,3,5-substituted pyrazole ring. J. Agric. Food Chem. 2006, 54, 3636–3640. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Cai, X.J.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, K.; Park, S.J.; Ahn, B.; Lee, J.C.; Cho, H.; Lee, K.I. Identification of antitumor activity of pyrazole oxime ethers. Bioorg. Med. Chem. Lett. 2005, 15, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.B.; Ivancic, W.A.; Saxena, A.M.; Allton, J.D.; O’Brien, G.K.; Suzuki, T.; Nokata, M. Direct photolysis of fenpyroximate in a buffered aqueous solution under a xenon lamp. J. Agric. Food Chem. 1995, 43, 513–518. [Google Scholar] [CrossRef]
- Motoba, K.; Nishizawa, H.; Suzuki, T.; Hamaguchi, H.; Uchida, M.; Funayama, S. Species-specific detoxification metabolism of fenpyroximate, a potent acaricide. Pestic. Biochem. Physiol. 2000, 67, 73–84. [Google Scholar] [CrossRef]
- Dai, H.; Xiao, Y.S.; Li, Z.; Xu, X.Y.; Qian, X.H. The thiazoylmethoxy modification on pyrazole oximes: Synthesis and insecticidal biological evaluation beyond acaricidal activity. Chin. Chem. Lett. 2014, 25, 1014–1016. [Google Scholar] [CrossRef]
- Dai, H.; Huang, J.; Jin, Z.; Cheng, X.; Huang, K.; Shi, Y.; Wang, Q.; Ling, Y. Synthesis and bioactivities of novel pyrazole oximes containing substituted thiadiazole moiety. Chin. J. Org. Chem. 2015, 35, 2617–2623. [Google Scholar] [CrossRef]
- Wang, S.L.; Shi, Y.J.; He, H.B.; Li, Y.; Li, Y.; Dai, H. Synthesis and bioactivity of novel pyrazole oxime derivatives containing oxazole ring. Chin. Chem. Lett. 2015, 26, 672–674. [Google Scholar] [CrossRef]
- Dai, H.; Li, G.; Chen, J.; Shi, Y.; Ge, S.; Fan, C.; He, H. Synthesis and biological activities of novel 1,3,4-thiadiazole-containing pyrazole oxime derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3818–3821. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Park, H.J.; Park, K.H.; Lee, K.I. Introduction of N-containing heterocycles into pyrazole by nucleophilic aromatic substitution. Synth. Commun. 2004, 34, 1541–1550. [Google Scholar] [CrossRef]
- Tanaka, A.; Terasawa, T.; Hagihara, H.; Sakuma, Y.; Ishibe, N.; Sawada, M.; Takasugi, H.; Tanaka, H. Inhibitors of acyl-CoA: Cholesterol O-acyltransferase. 2. Identification and structure-activity relationships of a novel series of N-alkyl-N-(heteroaryl-substituted benzyl)-N′-arylureas. J. Med. Chem. 1998, 41, 2390–2410. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Fang, Y.; Li, Y.; Chen, J.; Li, G.; Wang, Q.M.; Dai, H. Synthesis and biological activities of novel cyanoacrylate derivatives carrying 5-arylisoxazole group. Chem. J. Chin. Univ. 2017, 38, 421–428. [Google Scholar]
- Yu, X.L.; Liu, Y.X.; Li, Y.Q.; Wang, Q.M. Design, synthesis, and acaricidal/insecticidal activities of oxazoline derivatives containing a sulfur ether moiety. J. Agric. Food Chem. 2015, 63, 9690–9695. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.B.; Xia, R.; Luo, M.; Xu, X.Y.; Cheng, J.G.; Shao, X.S.; Li, Z. Design, synthesis, crystal structures, and insecticidal activities of eight-membered azabridge neonicotinoid analogues. J. Agric. Food Chem. 2014, 62, 381–390. [Google Scholar] [CrossRef] [PubMed]
| Compd. | Tetranychus cinnabarinus | Aphis medicaginis | |||
|---|---|---|---|---|---|
| 500 μg/mL | 100 μg/mL | 500 μg/mL | 100 μg/mL | 20 μg/mL | |
| 9a | 0 | — b | 90.52 ± 0.72 | 40.54 ± 1.22 | 0 |
| 9b | 0 | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 40.89 ± 0.57 |
| 9c | 0 | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 9d | 0 | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 60.36 ± 1.08 |
| 9e | 80.46 ± 0.65 a | 50.49 ± 1.78 | 100.00 ± 0.00 | 90.66 ± 0.53 | 50.67 ± 1.36 |
| 9f | 0 | — | 50.33 ± 1.59 | 0 | — |
| 9g | 0 | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 80.73 ± 0.71 |
| 9h | 0 | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 90.56 ± 0.82 |
| 9i | 0 | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 85.83 ± 0.69 |
| 9j | 0 | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 80.28 ± 1.21 |
| 9k | 0 | — | 100.00 ± 0.00 | 0 | — |
| 9l | 0 | — | 0 | — | — |
| 9m | 0 | — | 50.72 ± 1.37 | 0 | — |
| 9n | 0 | — | 0 | — | — |
| 9o | 0 | — | 0 | — | — |
| 9p | 0 | — | 0 | — | — |
| 9q | 50.38 ± 1.23 | 0 | 100.00 ± 0.00 | 0 | — |
| 9r | 0 | — | 100.00 ± 0.00 | 70.89 ± 1.25 | 0 |
| 9s | 0 | — | 100.00 ± 0.00 | 40.57 ± 0.68 | — |
| 9t | 0 | — | 100.00 ± 0.00 | 0 | — |
| 9u | 0 | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 90.78 ± 1.35 |
| 9v | 0 | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 90.62 ± 0.96 |
| 13a | 0 | — | 0 | — | — |
| 13b | 0 | — | 0 | — | — |
| 13c | 20.26 ± 1.61 | — | 100.00 ± 0.00 | 20.92 ± 0.52 | — |
| 13d | 0 | — | 0 | — | — |
| 13e | 0 | — | 0 | — | — |
| 13f | 0 | — | 100.00 ± 0.00 | 30.81 ± 1.47 | — |
| Fenpyroximate | 100.00 ± 0.00 | 100.00 ± 0.00 | — | — | — |
| Chlorantraniliprole | — | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| Compd. | Mythimna separata | Nilaparvata lugens | ||||
|---|---|---|---|---|---|---|
| 500 μg/mL | 100 μg/mL | 20 μg/mL | 500 μg/mL | 100 μg/mL | 20 μg/mL | |
| 9a | 0 | — b | — | 85.54 ± 1.32 | 0 | — |
| 9b | 90.37 ± 0.85 a | 0 | — | 0 | — | — |
| 9c | 100.00 ± 0.00 | 100.00 ± 0.00 | 40.57 ± 0.76 | 100.00 ± 0.00 | 0 | — |
| 9d | 60.29 ± 1.03 | 0 | — | 100.00 ± 0.00 | 0 | — |
| 9e | 50.55 ± 0.92 | 0 | — | 90.26 ± 1.45 | 75.23 ± 0.69 | 30.87 ± 1.43 |
| 9f | 80.76 ± 0.65 | 0 | — | 50.32 ± 1.21 | 0 | — |
| 9g | 80.32 ± 0.82 | 50.43 ± 0.73 | 0 | 80.79 ± 0.87 | 75.76 ± 0.75 | 0 |
| 9h | 100.00 ± 0.00 | 40.56 ± 0.47 | — | 95.23 ± 0.65 | 50.43 ± 1.58 | 0 |
| 9i | 100.00 ± 0.00 | 30.19 ± 1.58 | — | 100.00 ± 0.00 | 0 | — |
| 9j | 90.74 ± 0.57 | 0 | — | 90.86 ± 0.71 | 0 | — |
| 9k | 100.00 ± 0.00 | 100.00 ± 0.00 | 70.86 ± 0.63 | 100.00 ± 0.00 | 0 | — |
| 9l | 0 | — | — | 50.43 ± 0.54 | 0 | — |
| 9m | 0 | — | — | 80.31 ± 1.29 | 0 | — |
| 9n | 0 | — | — | 0 | — | — |
| 9o | 100.00 ± 0.00 | 0 | — | 0 | — | — |
| 9p | 100.00 ± 0.00 | 60.58 ± 1.21 | 0 | 0 | — | — |
| 9q | 100.00 ± 0.00 | 0 | — | 70.32 ± 0.86 | 0 | — |
| 9r | 100.00 ± 0.00 | 30.48 ± 0.65 | — | 100.00 ± 0.00 | 40.87 ± 0.73 | — |
| 9s | 100.00 ± 0.00 | 0 | — | 100.00 ± 0.00 | 0 | — |
| 9t | 100.00 ± 0.00 | 70.18 ± 1.43 | 30.27 ± 1.22 | 100.00 ± 0.00 | 30.75 ± 0.82 | — |
| 9u | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0 | — | — |
| 9v | 100.00 ± 0.00 | 50.21 ± 1.56 | 0 | 0 | — | — |
| 13a | 100.00 ± 0.00 | 40.43 ± 1.81 | — | 0 | — | — |
| 13b | 100.00 ± 0.00 | 30.61 ± 0.63 | — | 0 | — | — |
| 13c | 100.00 ± 0.00 | 0 | — | 20.68 ± 1.21 | — | — |
| 13d | 70.91 ± 0.89 | 0 | — | 100.00 ± 0.00 | 20.65 ± 0.53 | — |
| 13e | 100.00 ± 0.00 | 0 | — | 30.21 ± 1.12 | — | — |
| 13f | 100.00 ± 0.00 | 0 | — | 20.57 ± 0.43 | — | — |
| Pyridalyl | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | — | — | — |
| Abamectin | — | — | — | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Yao, W.; Fang, Y.; Sun, S.; Shi, Y.; Chen, J.; Jiang, G.; Shi, J. Design, Synthesis and Bioactivities of Novel Isoxazole-Containing Pyrazole Oxime Derivatives. Molecules 2017, 22, 2000. https://doi.org/10.3390/molecules22122000
Dai H, Yao W, Fang Y, Sun S, Shi Y, Chen J, Jiang G, Shi J. Design, Synthesis and Bioactivities of Novel Isoxazole-Containing Pyrazole Oxime Derivatives. Molecules. 2017; 22(12):2000. https://doi.org/10.3390/molecules22122000
Chicago/Turabian StyleDai, Hong, Wei Yao, Yuan Fang, Siyu Sun, Yujun Shi, Jia Chen, Guoqing Jiang, and Jian Shi. 2017. "Design, Synthesis and Bioactivities of Novel Isoxazole-Containing Pyrazole Oxime Derivatives" Molecules 22, no. 12: 2000. https://doi.org/10.3390/molecules22122000
APA StyleDai, H., Yao, W., Fang, Y., Sun, S., Shi, Y., Chen, J., Jiang, G., & Shi, J. (2017). Design, Synthesis and Bioactivities of Novel Isoxazole-Containing Pyrazole Oxime Derivatives. Molecules, 22(12), 2000. https://doi.org/10.3390/molecules22122000





