Update on Phytochemistry and Pharmacology of Naturally Occurring Resveratrol Oligomers
Abstract
:1. Introduction
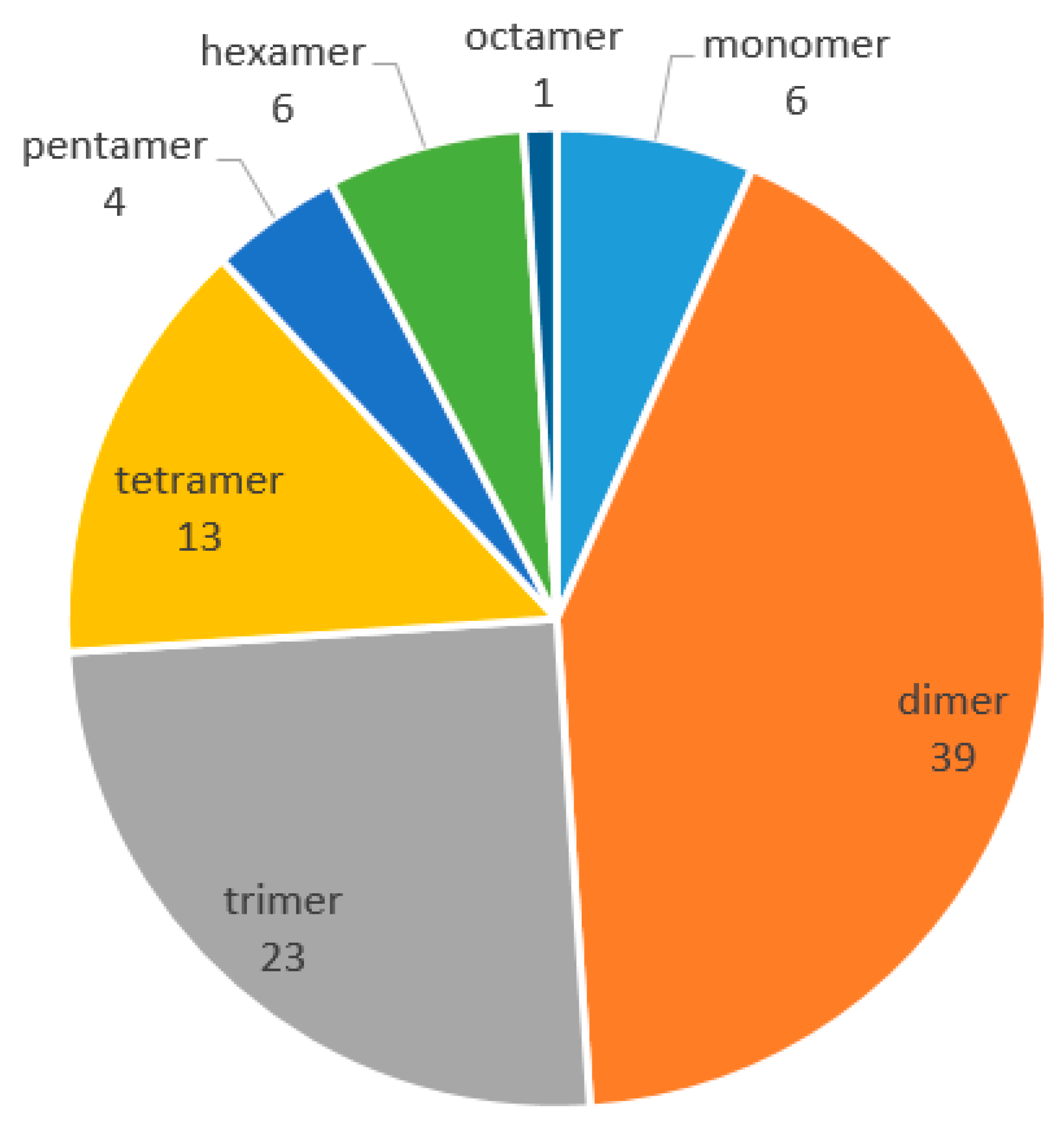
2. Phytochemistry
2.1. Resveratrol Monomers
2.2. Resveratrol Dimers
2.3. Resveratrol Trimers
2.4. Resveratrol Tetramers
2.5. Resveratrol Pentamers
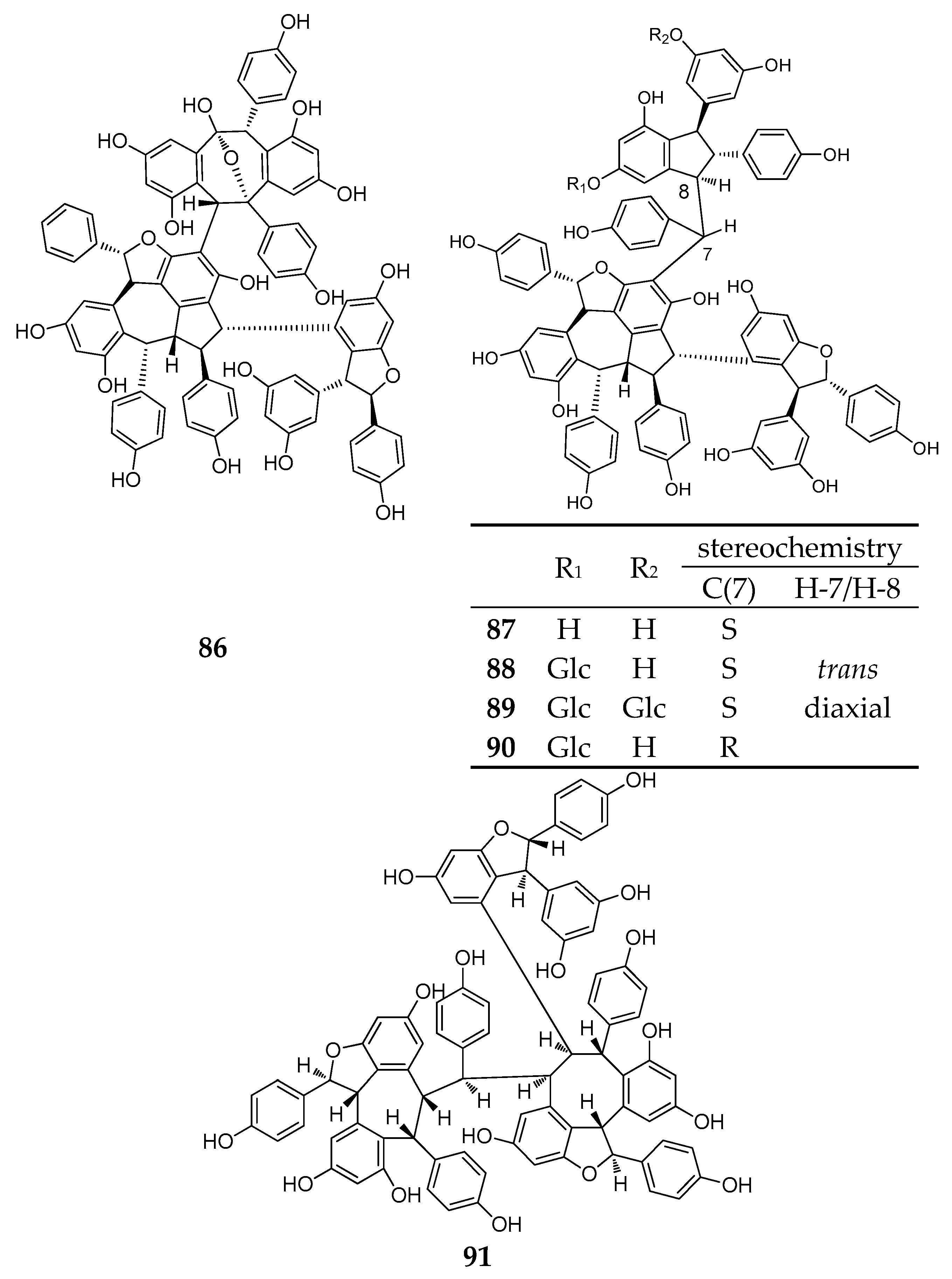
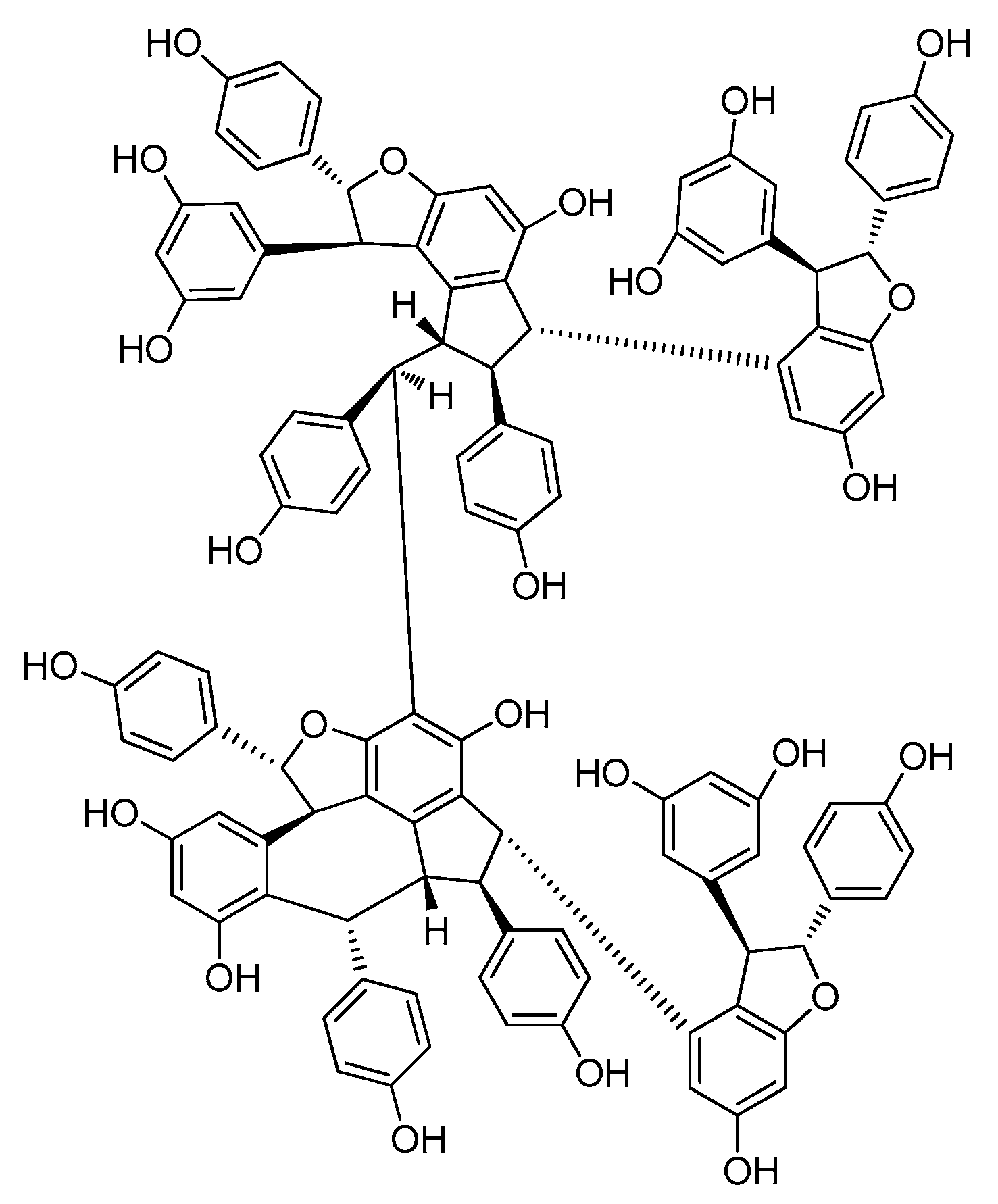
2.6. Resveratrol Hexamers
2.7. Resveratrol Heptamers
2.8. Resveratrol Octamers
3. Pharmacological Activities
3.1. Anti-Microbial Activities
3.2. Anti-Alzheimer’s Disease (AD)
3.3. Anti-Parkinson’s Disease (PD)
3.4. Antitumor Activity
3.5. Cardiovascular Protection
3.6. Liver-Protective Effect
3.7. Other Activities
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| No. | Chemical Component | Source | Part of Plant | Ref. |
|---|---|---|---|---|
| Resveratrol Monomer | ||||
| Moraceae | ||||
| 1 | cudrastilbene | Cudrania tricuspidata | roots | [19] |
| Leguminosae | ||||
| 2 | 3,5,3′-trihydroxy-4′-methoxy-5′-isopentenylstilbene | Arachis hypogaea | seeds | [20] |
| 3 | chiricanine B | Arachis hypogaea | seeds | [21] |
| 4 | arahypin-13 | Arachis hypogaea | seeds | [21] |
| 5 | arahypin-14 | Arachis hypogaea | seeds | [21] |
| 6 | arahypin-15 | Arachis hypogaea | seeds | [21] |
| Resveratrol Dimer | ||||
| Dipterocarpaceae | ||||
| 7 | vatalbinoside C | Vatica albiramis | stems | [22] |
| 8 | vatalbinoside D | Vatica albiramis | stems | [22] |
| 9 | vatalbinoside E | Vatica albiramis | stems | [22] |
| 10 | albiraminols B | Vatica albiramis | stems | [23] |
| 11 | vatalbinoside F | Vatica albiramis | stems | [23] |
| 12 | vaticahainols A | Vatica mangachapoi. | branches and twigs | [24] |
| 13 | vaticahainols B | Vatica mangachapoi. | branches and twigs | [24] |
| 14 | vaticahainols C | Vatica mangachapoi. | branches and twigs | [24] |
| 15 | vateriosides A | Vateria indica | leaves | [25] |
| 16 | roxburghiol A | Shorea roxburghii | roots | [26] |
| 17 | acuminatol | Shorea acuminata | stem barks | [27] |
| 18 | dipterocarpols A | Dipterocarpus alatus | stemwood | [28] |
| 19 | dipterocarpols B | Dipterocarpus alatus | stemwood | [28] |
| 20 | upunosides F | Upuna borneensis | leaves | [29] |
| 21 | upunosides G | Upuna borneensis | leaves | [29] |
| 22 | cordifoloside A | Shorea cordifolia | leaves | [30] |
| 23 | cordifoloside B | Shorea cordifolia | leaves | [30] |
| 24 | hopeasides D | Hopea parviflora | stems | [31] |
| 25 | heimiol B | Neobalanocarpus heimii | heartwood | [32] |
| Vitaceae | ||||
| 26 | amurensin O | Vitis amurensis | roots | [33] |
| Paeoniaceae | ||||
| 27 | (−)-7α,8α-cis-ε-viniferin | Paeonia lactiflora | seeds | [34] |
| Leguminosae | ||||
| 28 | arahypin 6 | Arachis hypogaea | seeds | [35] |
| 29 | arahypin 7 | Arachis hypogaea | seeds | [35] |
| 30 | arahypin-11 | Arachis hypogaea | seeds | [20] |
| 31 | arahypin-12 | Arachis hypogaea. | seeds | [20] |
| Gnetaceae | ||||
| 32 | macrostachyols C | Gnetum macrostachyum | roots | [36] |
| 33 | macrostachyols D | Gnetum macrostachyum | roots | [36] |
| 34 | gnemontanins A | Gnetum montanum | caulis | [37] |
| 35 | gnemontanins B | Gnetum montanum | caules | [37] |
| 36 | gnemontanins C | Gnetum montanum | caules | [37] |
| 37 | gnemontanins D | Gnetum montanum | caules | [37] |
| 38 | gnemontanins E | Gnetum montanum | caules | [37] |
| 39 | gnemontanins F | Gnetum montanum | caules | [37] |
| 40 | gnemontanins G | Gnetum montanum | caules | [37] |
| 41 | (−)-gnetuhainin P | Gnetum montanum | caules | [37] |
| 42 | (−)-gnetuhainin I | Gnetum montanum | caules | [37] |
| Cyperaceae | ||||
| 43 | longusol A | Cyperus longus | whole plant | [38] |
| 44 | longusol B | Cyperus longus | whole plant | [38] |
| 45 | longusol C | Cyperus longus | whole plant | [38] |
| Resveratrol Trimer | ||||
| Dipterocarpaceae | ||||
| 46 | malaysianol A | Dryobalanops aromatica | stem barks | [40] |
| 47 | malaysianol D | Dryobalanops beccarii | stem barks | [41] |
| 48 | hopeaside E | Hopea utilis | stems | [42] |
| 49 | hopeasides C | Hopea parviflora | stems | [30] |
| 50 | hopeachinols E | Hopea chinensis | stem barks | [43] |
| 51 | hopeachinols F | Hopea chinensis | stem barks | [43] |
| 52 | hopeachinol G | Hopea chinensis | stem barks | [43] |
| 53 | hopeachinols H | Hopea chinensis | stem barks | [43] |
| 54 | hopeachinols I | Hopea chinensis | stem barks | [43] |
| 55 | dipterocarpols C | Dipterocarpus alatus | stem wood | [28] |
| 56 | dipterocarpols D | Dipterocarpus alatus | stem wood | [28] |
| Vitaceae | ||||
| 57 | wenchowenol | Vitis wenchowensis | roots and stems | [44] |
| 58 | quinquangularol | Vitis quinquangularis | roots and stems | [45] |
| 59 | (Z)-cis-miyabenol C | Vitis vinifera | grapevine shoot | [46] |
| Paeoniaceae | ||||
| 60 | trans- suffruticosol D | Paeonia suffruticosa | seeds | [47] |
| 61 | cis-suffruticosol D | Paeonia suffruticosa | seeds | [47] |
| 62 | cis-gnetin H | Paeonia suffruticosa | seeds | [47] |
| Gnetaceae | ||||
| 63 | macrostachyol B | Gnetum macrostachyum | roots | [36] |
| 64 | gnetubrunol A | Gnetum brunonianum | roots | [48] |
| Polygonaceae | ||||
| 65 | rheumlhasol A | Rheum lhasaense | roots | [49] |
| 66 | rheumlhasol B | Rheum lhasaense | roots | [49] |
| Gramineae | ||||
| 67 | cystibenetrimerol A | Cynodon dactylon | dried grass | [50] |
| 68 | cystibenetrimerol B | Cynodon dactylon | dried grass | |
| Resveratrol Tetramer | ||||
| Dipterocarpaceae | ||||
| 69 | vatalbinoside A | Vatica albiramis | stems | [22] |
| 70 | vatalbinoside B | Vatica albiramis | stems | [22] |
| 71 | vaticanol L | Vatica chinensis | stems | [51] |
| 72 | vateriaphenol F | Vateria indica | leaves | [25] |
| 73 | vateriosides B | Vateria indica | leaves | [25] |
| 74 | heimiols C | Neobalanocarpus heimii | heartwood | [30] |
| 75 | heimiols D | Neobalanocarpus heimii | heartwood | [30] |
| 76 | heimiols E | Neobalanocarpus heimii | heartwood | [30] |
| 77 | malaysianol B | Dryobalanops lanceolata | stem barks | [52] |
| 78 | malaysianol C | Dryobalanops lanceolata | stem barks | [53] |
| Gnetaceae | ||||
| 79 | macrostachyol A | Gnetum macrostachyum. | roots | [36] |
| Vitaceae | ||||
| 80 | cajyphenol A | Cayratia japonica | stems | [54] |
| 81 | cajyphenol B | Cayratia japonica | stems | [54] |
| Resveratrol Pentamer | ||||
| Dipterocarpaceae | ||||
| 82 | hopeaside F | Hopea utilis | stems | [42] |
| 83 | hopeasides A | Hopea parviflora | stems | [29] |
| 84 | hopeasides B | Hopea parviflora | stems | [29] |
| 85 | upunosides E | Upuna borneensis | leaves | [32] |
| Resveratrol Hexamer | ||||
| Dipterocarpaceae | ||||
| 86 | albiraminols A | Vatica albiramis | stems | [22] |
| 87 | vatcaside M | Vatica bantamensis; | leaveas | [55] |
| Vatica chinensis; | ||||
| Vatica chinensis | ||||
| 88 | vatcasides E | Vatica bantamensis, | leaveas | [55] |
| Vatica chinensis; | ||||
| 89 | vatcasides F | Vatica bantamensis, | leaveas | [55] |
| Vatica chinensis; | ||||
| 90 | vatcasides G | Vatica bantamensis, | leaveas | [55] |
| Vatica chinensis; | ||||
| Vitaceae | ||||
| 91 | viniphenol A | Vitis vinifera | vine stalks | [56] |
| Resveratrol Octamer | ||||
| Dipterocarpaceae | ||||
| 92 | upunaphenol Q | Upuna borneensis | leaves | [59] |
References
- Lin, M.; Yao, C.S. Natural Oligostilbenes. Stud. Nat. Prod. Chem. 2006, 33, 601–644. [Google Scholar]
- Wang, Z.P.; Chen, Y.P.; Liang, S.; Wang, J.; Zheng, H.H.; Hong-Hua, W.U. Resveratrol Oligomers from Nardostachys chinensis Batal. Nat. Prod. Res. Dev. 2014, 26, 1548–1551. [Google Scholar]
- Yang, S.L. Terpenoids from the Roots and Rhizomes of Nardostachys chinensis. J. Nat. Prod. 2005, 68, 1131. [Google Scholar]
- Appanah, S.; Turnbull, J.M. A Review of Dipterocarps: Taxonomy, Ecology and Silviculture; CIFOR: Bogor, Indonesia, 1998. [Google Scholar]
- Jang, M.H.; Piao, X.L.; Kim, H.Y.; Cho, E.J.; Baek, S.H.; Kwon, S.W.; Park, J.H. Resveratrol oligomers from Vitis amurensis attenuate beta-amyloid-induced oxidative stress in PC12 cells. Biol. Pharm. Bull. 2007, 30, 1130. [Google Scholar] [CrossRef] [PubMed]
- Nazri, N.A.A.M.; Ahmat, N.; Abdullah, M.; Sidik, N.J.; Johari, S.A.T.T. Antioxidant, antimicrobial and cytotoxic activities of resveratrol oligomers of shorea macroptera dyer. Aust. J. Basic. Appl. Sci. 2012, 6, 431–436. [Google Scholar]
- Gonzalez-Sarrias, A.; Gromek, S.; Niesen, D.; Seeram, N.P.; Henry, G.E. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food. Chem. 2011, 59, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Hien, T.T.; Oh, W.K.; Quyen, B.T.; Dao, T.T.; Yoon, J.H.; Yun, S.Y.; Kang, K.W. Potent vasodilation effect of amurensin G is mediated through the phosphorylation of endothelial nitric oxide synthase. Biochem. Pharmacol. 2012, 84, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhang, H.J.; Xuan, L.J.; Zhang, J.; Xu, Y.M.; Bai, D.L. Stilbenoids: Chemistry and bioactivities. Stud. Nat. Prod. Chem. 2008, 34, 453–646. [Google Scholar]
- Shen, T.; Wang, X.N.; Lou, H.X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Fulvio, M.; Mirko, D.R.; Panagiotis, A.; Luigi, B. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar]
- Tetsuro, I. Structures of Oligostilbenoids in Dipterocarpaceaeous Plants and Their Biological Activities. Yakugaku Zasshi 2011, 131, 93–100. [Google Scholar]
- Niesen, D.B.; Hessler, C.; Seeram, N.P. Beyond resveratrol: A review of natural stilbenoids identified from 2009–2013. J. Berry Res. 2013. [Google Scholar] [CrossRef]
- Lim, K.G.; Gray, A.I.; Anthony, N.G.; Mackay, S.P.; Pyne, S.; Pyne, N.J. Resveratrol and its oligomers: Modulation of sphingolipid metabolism and signaling in disease. Arch. Toxicol. 2014, 88, 2213–2232. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.Q.; Di, J.M.; Luo, Y.; Cheng, K.J.; Wei, X.; Shi, Z. Resveratrol oligomers for the prevention and treatment of cancers. Oxid. Med. Cell. Longev. 2014, 2014, 765832. [Google Scholar] [CrossRef] [PubMed]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R. Chemistry and Biology of Resveratrol-Derived Natural Products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Yao, C.S. Naturally active oligostilbenes. J. Asian Nat. Prod. Res. 2016, 18, 376–407. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Inaoka, P.T. Gnetin-C and other resveratrol oligomers with cancer chemopreventive potential. Ann. N. Y. Acad. Sci. 2017, 1403, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.G.; Shi, L.L.; Ying, Y.M.; Hou, X.R.; Zhan, Z.J. ChemInform Abstract: A New Prenylated Stilbene Derivative from the Roots of Cudrania tricuspidata. ChemInform 2013, 44, 285–286. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, J.; Huang, D. New stilbenoids isolated from fungus-challenged black skin peanut seeds and their adipogenesis inhibitory activity in 3T3-L1 cells. J. Agric. Food. Chem. 2013, 61, 4155–4161. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S.; Krausert, N.M.; Gloer, J.B. New Monomeric Stilbenoids from Peanut (Arachis hypogaea) Seeds Challenged by an Aspergillus flavus Strain. J. Agric. Food. Chem. 2016, 64, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Ito, T.; Ohguchi, K.; Nasu, M.; Masuda, Y.; Oyama, M.; Nozawa, Y.; Ito, M.; Iinuma, M. Resveratrol Oligomers from Vatica albiramis. J. Nat. Prod. 2010, 73, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Ito, T.; Oyama, M.; Sawa, R.; Takahashi, Y.; Iinuma, M. Resveratrol derivatives from Vatica albiramis. ChemInform 2011, 59, 452–457. [Google Scholar] [CrossRef]
- Qin, Y.H.; Zhang, J.; Cui, J.T.; Guo, Z.K.; Jiang, N.; Tan, R.X.; Ge, H.M. Oligostilbenes from Vatica mangachapoi with xanthine oxidase and acetylcholinesterase inhibitory activities. RSC Adv. 2011, 1, 135. [Google Scholar] [CrossRef]
- Ito, T.; Masuda, Y.; Abe, N.; Oyama, M.; Sawa, R.; Takahashi, Y.; Chelladurai, V.; Iinuma, M. Chemical constituents in the leaves of Vateria indica. Chem. Pharm. Bull. 2010, 58, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Patcharamun, W.; Sichaem, J.; Siripong, P.; Khumkratok, S.; Jong-aramruang, J.; Tip-pyang, S. A new dimeric resveratrol from the roots of Shorea roxburghii. Fitoterapia 2011, 82, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Din, L.B.; Sahidin, I.; Hashim, S.F.; Ibrahim, N.; Zakaria, Z.; Yaacob, W.A. Acuminatol and other antioxidative resveratrol oligomers from the stem bark of Shorea acuminata. Molecules 2012, 17, 9043–9055. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Jiang, R.; Wang, G.; Jiao, R.H.; Tancharoen, C.; Sudto, K.; Vajarothai, S.; Hannongbua, S.; Ge, H.M.; Tan, R.X. Oligostilbenoids with acetylcholinesterase inhibitory activity from Dipterocarpus alatus. Planta Med. 2014, 80, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ito, H.; Iinuma, M. Absolute configuration of resveratrol oligomer glucosides isolated from the leaves of Upuna borneensis. Phytochem. Lett. 2017, 20, 26–31. [Google Scholar] [CrossRef]
- Ito, T.; Nishiya, K.; Oyama, M.; Tanaka, T.; Murata, J.; Darnaedi, D.; Iinuma, M. Novel isolation of resveratrol dimer O-glucosides with enantiomeric aglycones from the leaves of Shorea cordifolia. Phytochem. Lett. 2013, 6, 667–670. [Google Scholar] [CrossRef]
- Abe, N.; Ito, T.; Oyama, M.; Sawa, R.; Takahashi, Y.; Chelladurai, V.; Iinuma, M. ChemInform Abstract: Occurrence of C-Glucoside of Resveratrol Oligomers in Hopea parviflora. ChemInform 2011, 42, 239–248. [Google Scholar] [CrossRef]
- Bayach, I.; Manshoor, N.; Sanchogarcía, J.C.; Choudhary, M.I.; Trouillas, P.; Weber, J.F. Oligostilbenoids from the Heartwood of N. Heimii: Role of Non-Covalent Association in their Biogenesis. Chem.—Asian J. 2015, 10, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.S.; Huang, K.S.; Lin, M.; Yang, Q.Y. A new stilbene dimer from Vitis amurensis. J Asian Nat. Prod. Res. 2013, 15, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Choi, Y.H.; Cha, M.R.; Kim, Y.S.; Yon, G.H.; Hong, K.S.; Park, W.K.; Kim, Y.H.; Ryu, S.Y. In vitro BACE-1 inhibitory activity of resveratrol oligomers from the seed extract of Paeonia lactiflora. Planta Med. 2010, 77, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S.; Neff, S.A.; Gloer, J.B. New dimeric stilbenoids from fungal-challenged peanut (Arachis hypogaea) seeds. J. Agric. Food. Chem. 2010, 58, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Sri-in, P.; Sichaem, J.; Siripong, P.; Tip-pyang, S. Macrostachyols A-D, new oligostilbenoids from the roots of Gnetum macrostachyum. Fitoterapia 2011, 82, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.M.; Jiang, K.; Qu, S.J.; Luo, H.F.; Tan, J.J.; Tan, C.H. Structurally diverse stilbene dimers from Gnetum montanum Markgr.: Studies on the 1H chemical shift differences between dimeric stilbene epimers correlating to the relative configurations. RSC Adv. 2016, 6, 50083–50090. [Google Scholar] [CrossRef]
- Morikawa, T.; Xu, F.; Matsuda, H.; Yoshikawa, M. ChemInform Abstract: Structures of Novel Norstilbene Dimer, Longusone A, and Three New Stilbene Dimers, Longusols A, B, and C, with Antiallergic and Radical Scavenging Activities from Egyptian Natural Medicine Cyperus longus. ChemInform 2011, 58, 1379–1385. [Google Scholar] [CrossRef]
- Ashton, P.S. Flora Malesiana, I, Spermatophyta; Springer: Berlin/Heidelberg, Germany, 1982; pp. 182–197. [Google Scholar]
- Wibowo, A.; Ahmat, N.; Hamzah, A.S.; Sufian, A.S.; Ismail, N.H.; Ahmad, R.; Jaafar, F.M.; Takayama, H. Malaysianol A, a new trimer resveratrol oligomer from the stem bark of Dryobalanops aromatica. Fitoterapia 2011, 82, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Ahmat, N.; Hamzah, A.S.; Latif, F.A.; Norrizah, J.S.; Khong, H.Y.; Takayama, H. Identification and biological activity of secondary metabolites from Dryobalanops beccarii. Phytochem. Lett. 2014, 9, 117–122. [Google Scholar] [CrossRef]
- Ito, T.; Hoshino, R.; Iinuma, M. Absolute Configuration of Resveratrol Oligomers Isolated from Hopea utilis. Helv. Chim. Acta 2015, 98, 32–46. [Google Scholar] [CrossRef]
- Cheng, Y.; Jiang, R.; Huang, W.; Wei, W.; Chen, C.; Tan, R.; Ge, H. Hopeachinols E–K, novel oligostilbenoids from the stem bark of Hopea chinensis. RSC Adv. 2014, 4, 28901–28907. [Google Scholar] [CrossRef]
- Gu, B.; Xu, Y.; He, S. A new resveratrol trimer from the roots and stems of Vitis wenchowensis. Molecules 2013, 18, 7486–7491. [Google Scholar] [CrossRef] [PubMed]
- Wu, X. A new Antioxidative Resveratrol Trimer from the Roots and Stems of Vitis quinquangularis. Rec. Nat. Prod. 2016, 10, 349. [Google Scholar]
- Chaher, N.; Arraki, K.; Dillinseger, E.; Temsamani, H.; Bernillon, S.; Pedrot, E.; Delaunay, J.C.; Merillon, J.M.; Monti, J.P.; Izard, J.C.; et al. Bioactive stilbenes from Vitis vinifera grapevine shoots extracts. J. Sci. Food Agric. 2014, 94, 951–954. [Google Scholar] [CrossRef] [PubMed]
- He, C.N.; Peng, Y.; Xu, L.J.; Liu, Z.A.; Gu, J.; Zhong, A.G.; Xiao, P.G. Three new oligostilbenes from the seeds of Paeonia suffruticosa. ChemInform 2010, 58, 843–847. [Google Scholar] [CrossRef]
- Yao, C.S.; Lin, M.; Yang, Q.Y. A new resveratrol trimer derivative from Gnetum brunonianum. J. Asian Nat. Prod. Res. 2012, 14, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.B.; Hu, L.; Hu, Q.; Chen, N.N.; Yang, Q.S.; Wang, F.F. New resveratrol oligomer derivatives from the roots of Rheum lhasaense. Molecules 2013, 18, 7093–7102. [Google Scholar] [CrossRef] [PubMed]
- Li, B.J.; Liu, Y.; Gu, A.T.; Zhang, Q.; Chen, L.; Wang, S.M.; Wang, F. Two new stilbene trimers from Cynodon dactylon. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Iinuma, M. Occurrence of non-heterocyclic resveratrol tetramer in Vatica chinensis. Phytochem. Lett. 2016, 15, 37–41. [Google Scholar] [CrossRef]
- Wibowo, A.; Ahmat, N.; Hamzah, A.S.; Low, A.L.M.; Mohamad, S.A.S.; Khong, H.Y.; Sufian, A.S.; Manshoor, N.; Takayama, H. Malaysianol B, an oligostilbenoid derivative from Dryobalanops lanceolata. Fitoterapia 2012, 83, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Ahmat, N.; Wibowo, A.; Mohamad, S.A.; Low, A.L.; Sufian, A.S.; Yusof, M.I.; Latip, J. A new symmetrical tetramer oligostilbenoid containing tetrahydrofuran ring from the stem bark of Dryobalanops lanceolata. J. Asian Nat. Prod. Res. 2014, 16, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Ma, X.F.; Song, X.H.; Wang, M.Y.; Liu, H.W. Two New Resveratrol Tetramers Isolated from Cayratia japonica (Thunb.) Gagn. with Strong Inhibitory Activity on Fatty Acid Synthase and Antioxidant Activity. Chem. Biodivers. 2010, 7, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Hara, Y.; Kubota, Y.; Sawa, R.; Iinuma, M. Absolute structure of resveratrol hexamers in Dipterocarpaceaeous plants. Tetrahedron 2016, 72, 891–899. [Google Scholar] [CrossRef]
- Papastamoulis, Y.; Richard, T.; Nassra, M.; Badoc, A.; Krisa, S.; Harakat, D.; Monti, J.P.; Merillon, J.M.; Waffo-Teguo, P. Viniphenol A, a complex resveratrol hexamer from Vitis vinifera stalks: Structural elucidation and protective effects against amyloid-beta-induced toxicity in PC12 cells. J. Nat. Prod. 2014, 77, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanaka, T.; Iinuma, M.; Nakaya, K.; Takahashi, Y.; Sawa, R.; Murata, J.; Darnaedi, D. Three New Resveratrol Oligomers from the Stem Bark of Vatica pauciflora. J. Nat. Prod. 2004, 67, 932. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanaka, T.; Nakaya, K.I.; Iinuma, M.; Takahashi, Y.; Naganawa, H.; Ohyama, M.; Nakanishi, Y.; Bastow, K.F.; Lee, K.H. A novel bridged stilbenoid trimer and four highly condensed stilbenoid oligomers in Vatica rassak. Tetrahedron 2001, 57, 7309–7321. [Google Scholar] [CrossRef]
- Ito, T.; Ito, H.; Nehira, T.; Sawa, R.; Iinuma, M. Structure elucidation of highly condensed stilbenoids: Chiroptical properties and absolute configuration. Tetrahedron 2014, 70, 5640–5649. [Google Scholar] [CrossRef]
- Ito, T.; Tanaka, T.; Iinuma, M.; Nakaya, K.I.; Takahashi, Y.; Sawa, R.; Naganawa, H.; Chelladurai, V. Two new oligostilbenes with dihydrobenzofuran from the stem bark of Vateria indica. Tetrahedron 2003, 59, 1255–1264. [Google Scholar] [CrossRef]
- Seo, E.K.; Kinghorn, A.D. Bioactive constituents of the family differocarpaceae. Stud. Nat. Prod. Chem. 2000, 23, 531–561. [Google Scholar]
- Suzuki, K.; Shimizu, T.; Kawabata, J.; Mizutani, J. New 3,5,4′-Trihydroxystilbene (Resveratrol) Oligomers from Nees var. (Franchet) T. Koyama (Cyperaceae). Agric. Biol. Chem. 1987, 51, 1003–1008. [Google Scholar]
- Ngoc, T.M.; Hung, T.M.; Thuong, P.T.; Na, M.; Kim, H.; Ha, d.T.; Min, B.S.; Minh, P.T.; Bae, K. Inhibition of human low density lipoprotein and high density lipoprotein oxidation by oligostilbenes from rhubarb. Biol. Pharm. Bull. 2008, 31, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Akao, Y.; Yi, H.; Ohguchi, K.; Matsumoto, K.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Antitumor effect of resveratrol oligomers against human cancer cell lines and the molecular mechanism of apoptosis induced by vaticanol C. Carcinogenesis 2003, 24, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Zain, W.Z.W.M.; Ahmat, N.; Norizan, N.H.; Nazri, N.A.A.M. The evaluation of antioxidant, antibacterial and structural identification activity of trimer resveratrol from Malaysia's dipterocarpaceae. Aust. J Basic Appl. Sci. 2011, 5, 926–929. [Google Scholar]
- Basri, D.F.; Luoi, C.K.; Azmi, A.M.; Latip, J. Evaluation of the Combined Effects of Stilbenoid from Shorea gibbosa and Vancomycin against Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceuticals 2012, 5, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Lee, J.H.; Shi, Y.R.; Sang, W.J.; Cho, M.H.; Lee, J. Inhibition of Pseudomonas aeruginosa and Escherichia coli O157:H7 Biofilm Formation by Plant Metabolite ε-Viniferin. J. Agric. Food. Chem. 2013, 61, 7120. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Ryu, S.Y.; Cho, M.H.; Lee, J. Resveratrol oligomers inhibit biofilm formation of Escherichia coli O157:H7 and Pseudomonas aeruginosa. J. Nat. Prod. 2014, 77, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ito, T.; Nakaya, K.I. Six New Heterocyclic Stilbene Oligomers from Stem Bark of Shorea hemsleyana. Heterocycles 2001, 55, 729–740. [Google Scholar] [CrossRef]
- Hernandez, C.; Villaseñor, I.M.; Schroeder, F.C.; Paulus, H.; Clardy, J. Stilbenoids from Hopea acuminata. J. Herbs Spices Med. Plants 2016, 22, 92–104. [Google Scholar] [CrossRef]
- Chen, X.; Qiao, H.; Liu, T.; Yang, Z.; Xu, L.; Xu, Y.; Ge, H.M.; Tan, R.X.; Li, E. Inhibition of herpes simplex virus infection by oligomeric stilbenoids through ROS generation. Antivir. Res. 2012, 95, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer9s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Riviere, C.; Papastamoulis, Y.; Fortin, P.Y.; Delchier, N.; Andriamanarivo, S.; Waffo-Teguo, P.; Kapche, G.D.; Amira-Guebalia, H.; Delaunay, J.C.; Merillon, J.M.; et al. New stilbene dimers against amyloid fibril formation. Bioorg. Med. Chem. Lett. 2010, 20, 3441–3443. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, T.; Gao, Y.; Xu, J.; Jiang, C.; Wang, G.; Bu, G.; Xu, H.; Chen, H.; Zhang, Y.W. The resveratrol trimer miyabenol C inhibits β-secretase activity and β-amyloid generation. PLoS ONE 2015, 10, e0115973. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Dawson, V.L. Molecular pathways of neurodegeneration in Parkinson‘s disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Temsamani, H.; Krisa, S.; Decossas-Mendoza, M.; Lambert, O.; Mérillon, J.-M.; Richard, T. Piceatannol and Other Wine Stilbenes: A Pool of Inhibitors against α-Synuclein Aggregation and Cytotoxicity. Nutrients 2016, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, H.; Moriyama, M.; Ninomiya, K.; Morikawa, T.; Hayakawa, T. Inhibitory Effects of Oligostilbenoids from the Bark of Shorea roxburghii on Malignant Melanoma Cell Growth: Implications for Novel Topical Anticancer Candidates. Biol. Pharm. Bull. 2016, 39, 1675. [Google Scholar] [CrossRef] [PubMed]
- Almosnid, N.M.; Gao, Y.; He, C.; Park, H.S.; Altman, E. In vitro antitumor effects of two novel oligostilbenes, cis-and trans-suffruticosol D, isolated from Paeonia suffruticosa seeds. Int. J. Oncol. 2016, 48, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.T.; Hwang, T.L.; Huang, Y.L.; Lin, C.F.; Wu, W.B. Vitisin B, a resveratrol tetramer, inhibits migration through inhibition of PDGF signaling and enhancement of cell adhesiveness in cultured vascular smooth muscle cells. Toxicol. Appl. Pharmacol. 2011, 256, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Kamarozaman, A.S.; Latip, J.; Syah, Y.M.; Rajab, N.; Jaloh, A. Oligostilbenoids fromVatica paucifloraand the Oxidative Effect on Chang Cells. J. Phys. Conf. Ser. 2013, 423, 012045. [Google Scholar] [CrossRef]
- Ninomiya, K.; Chaipech, S.; Kunikata, Y.; Yagi, R.; Pongpiriyadacha, Y.; Muraoka, O.; Morikawa, T. Quantitative Determination of Stilbenoids and Dihydroisocoumarins in Shorea roxburghiiand Evaluation of Their Hepatoprotective Activity. Int. J. Mol. Sci. 2017, 18, 452. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoon, K.D.; Lee, M.; Cho, Y.; Choi, G.; Jang, H.; Kim, B.S.; Jung, D.H.; Oh, J.G.; Kim, G.W. Identification of a resveratrol tetramer as a potent hepatitis C virus helicase inhibitor. Br. J. Pharmacol. 2016, 173, 191–211. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, M.Y.; Guo, Y. Virtual analysis of interaction of resveratrol oligomer with Fos/Jun and its analgesic activity in mice. Chin. J. New Drug 2014, 23, 80–85. [Google Scholar]
- Morikawa, T.; Chaipech, S.; Matsuda, H.; Hamao, M.; Umeda, Y.; Sato, H.; Tamura, H.; Kon‘i, H.; Ninomiya, K.; Yoshikawa, M.; et al. Antidiabetogenic oligostilbenoids and 3-ethyl-4-phenyl-3,4-dihydroisocoumarins from the bark of Shorea roxburghii. Bioorg. Med. Chem. 2012, 20, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Chaipech, S.; Matsuda, H.; Hamao, M.; Umeda, Y.; Sato, H.; Tamura, H.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; et al. Anti-hyperlipidemic constituents from the bark of Shorea roxburghii. J. Nat. Med. 2012, 66, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Hu, D.; Hou, L.B.; Song, L.Y.; Zhang, Y.J.; Xie, Y.; Tian, L.W. Phenolic Compounds from the Rhizomes of Smilax china L. and Their Anti-Inflammatory Activity. Molecules 2017, 22, 515. [Google Scholar] [CrossRef] [PubMed]
- Sotheeswaran, S.; Pasupathy, V. Distribution of resveratrol oligomers in plants. Phytochemistry 1993, 32, 1083–1092. [Google Scholar] [CrossRef]











© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Zhou, Q.; Li, P.; Wang, Z.; Liu, S.; He, C.; Zhang, C.; Xiao, P. Update on Phytochemistry and Pharmacology of Naturally Occurring Resveratrol Oligomers. Molecules 2017, 22, 2050. https://doi.org/10.3390/molecules22122050
Shen J, Zhou Q, Li P, Wang Z, Liu S, He C, Zhang C, Xiao P. Update on Phytochemistry and Pharmacology of Naturally Occurring Resveratrol Oligomers. Molecules. 2017; 22(12):2050. https://doi.org/10.3390/molecules22122050
Chicago/Turabian StyleShen, Jie, Qiang Zhou, Pei Li, Zhiqiang Wang, Shuangshuang Liu, Chunnian He, Chunhong Zhang, and Peigen Xiao. 2017. "Update on Phytochemistry and Pharmacology of Naturally Occurring Resveratrol Oligomers" Molecules 22, no. 12: 2050. https://doi.org/10.3390/molecules22122050
APA StyleShen, J., Zhou, Q., Li, P., Wang, Z., Liu, S., He, C., Zhang, C., & Xiao, P. (2017). Update on Phytochemistry and Pharmacology of Naturally Occurring Resveratrol Oligomers. Molecules, 22(12), 2050. https://doi.org/10.3390/molecules22122050





