Abstract
In order to discover novel eco-friendly lead compounds for plant pathogenic fungi control, a series of benzaldehyde thiosemicarbazide derivatives with a piperidine moiety have been designed and synthesized. Fungicidal activities of all the synthesized compounds were evaluated in vitro. The results indicated that all the title compounds exhibited moderate to good fungicidal activities. Compound 3b displayed excellent activities against Pythium aphanidermatum, Rhizoctonia solani, Valsa mali, and Gaeu-mannomyces graminsis, with EC50 values lower than 10 μg/mL. Especially, in the case of Pythium aphanidermatum, its activity (EC50 = 1.6 μg/mL) is superior to the commercial azoxystrobin (EC50 = 16.9 μg/mL) and close to fluopicolide (EC50 = 1.0 μg/mL). Initial structure–activity relationship (SAR) analysis showed that the heterocyclic piperidine group can influence the biological activities of the title compounds significantly. The fungicidal activity of compounds with piperidine is better than that of compounds without piperidine. The highly-active compound 3b, with its simple structure and easy synthetic route, is worthy to be further studied as a new lead fungicide.
1. Introduction
Plant diseases can significantly affect the yields of crops around the world, with losses up to 25% [1], as well as depreciate the quality and shorten the storage time of the fruits and vegetables [2]. Moreover, some fungi can harm human and animal health due to their mycotoxins [3]. Therefore, fungicide must be used to control fungal plant diseases. However, the extensive use of pesticides has led to residue, resistance, and resurgence, known as “3R” problems. Thus, developing efficient, low-toxicity, environment-friendly pesticides is particularly important.
Natural products, with the characteristics of lower toxicity and less environmental pollution, are often used as lead structures for the discovery of novel green pesticides [4]. Benzaldehyde and their derivatives widely exist in bitter almond oil, walnut oil, orange blossom oil, cinnamon oil, and other natural essential oils. As a spice with an annual demand of about 7000 tons [5], benzaldehyde is widely used in food, beverages, tobacco, and cosmetics. It also play important roles in medical chemistry and agrochemicals due to its derivatives’ (Figure 1) close association with various types of biological properties, such as antibacterial [6], antifungal [7], antioxidant [8], antitrypsnosomal [9], antiviral [10] functions, and so on [7,8]. Particularly, benzaldehyde thiosemicarbazide derivatives have been extensively applied to medicine, materials, pesticides, and other fields thanks to their good bioactivities (Figure 1A–C) [11,12,13,14,15,16,17]. Our team is also devoted to the structure modification of benzaldehyde thiosemicarbazide (Figure 1D) and has found that some analogues showed tyrosinase inhibitor activities in a previous study [18].
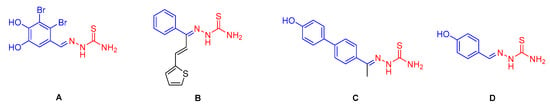
Figure 1.
The structure of the benzaldehyde thiosemicarbazide derivatives. (A) Anticancer activity; (B) antifungal activity; (C,D) tyrosinase inhibitor.
Heterocyclic compounds have received considerable attention in recent years owing to their broad bioactivities and have become of significant importance in industry and biology [19]. As a saturated heterocyclic, piperidine is a cyclic secondary amine that has been frequently reported for its multiple activities, such as anti-HIV [20], histamine H3R ligands (Figure 2A) [21], antimalarial [22], antimicrobial [23], antifungal (Figure 2B) [24], anticoagulant [25], and other biological properties [26]. For instance, oxathiapiprolin (Figure 2C), the first member of the piperidinyl thiazole isoxazoline fungicides developed globally as DuPont™ Zorvec™, can control plant diseases caused by oomycete pathogens in different stages of the pathogen’s life cycle at extremely low concentrations [27].
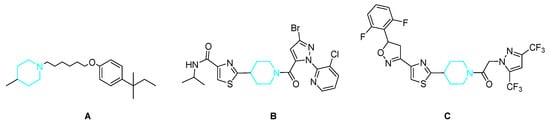
Figure 2.
The structure of the piperidine derivatives. (A) Histamine H3R ligands; (B) antifungal activity; (C) fungicide oxathiapiprolin.
In the view of the need of efficient and eco-friendly fungicidal lead compounds, a series of thiosemicarbazone analogues containing heterocyclic a piperidine moiety were designed and synthesized via linking active substructures with the aim to obtain a new prospective lead with a simple structure and good activities. The design strategy of the title compounds are shown in Figure 3. All the compounds were evaluated for their activities against six plant pathogenic fungi. In addition, the initial SAR analysis was also reported in the present work.
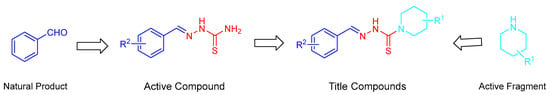
Figure 3.
Design strategy of the title compounds thiosemicarbazide derivatives containing piperidine fragments.
2. Results and Discussion
2.1. Chemistry
The synthetic route for the intermediates and benzaldehyde thiosemicarbazide analogues is illustrated in Scheme 1 and Scheme 2. Intermediate 1 was obtained from substituted piperidine and N,N′-thiocarbonyldiimidazole via a nucleophilic substitution reaction at room temperature, and then reacted with hydrazine hydrate to afford the key intermediate 2. The temperature had an important influence on yield of intermediate 2, and room temperature is favorable for the reaction. C–N bonds connected with imidazole cleavage easily compared to C–N bonds connected with piperidine, intermediate 2 was obtained when intermediate 1 was reacted with hydrazine hydrate under room temperature conditions. Double addition was taken place due to double C–N bonds being activated under reflux conditions. When intermediate 1 was reacted with hydrazine hydrate under reflux conditions, instead of intermediate 2, thiocarbohydrazide was obtained (Scheme 1).
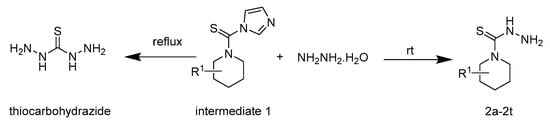
Scheme 1.
Reaction route of intermediate 1 and hydrazine hydrate at different temperatures.
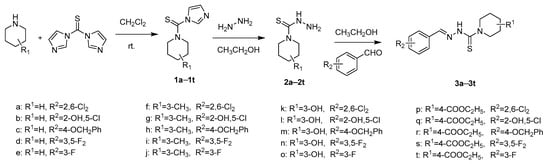
Scheme 2.
Synthetic route of the title compounds 3a–3t.
The title compounds 3a–3t were prepared from intermediate 2 followed by a condensation reaction with substituted benzaldehyde in ethanol as the solvent (Scheme 2).
Compounds 3u–3y were prepared from compounds 3p–3t through the hydrolysis reaction at room temperature in the NaOH aqueous solution (Scheme 3).
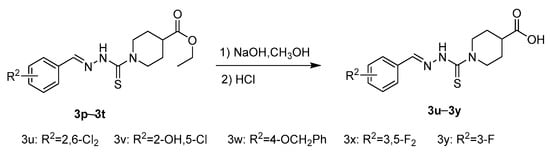
Scheme 3.
Synthetic route of the title compounds 3u–3y.
The structures of all synthesized benzaldehyde thiosemicarbazide analogues 3a–3y were confirmed by IR, 1H-NMR, 13C-NMR, DEPT-135, elemental analysis, or HR-ESI-MS. Their physical and chemical properties and structure characterization were described in Section 3.2. In the IR spectra, the analogues showed strong absorptions around 3300 cm−1 due to the N–H stretching vibration. A strong band at about 1700 cm−1 was detected because of the C=S stretching vibration. A weak band at about 1650 cm−1 was detected because of the C=N stretching vibration.
In the 1H-NMR spectrum of benzaldehyde thiosemicarbazide analogues, a wide single peak in the δ 11.0~11.2 ppm chemical shift range was observed due to the presence of NH protons. The protons of N=CH were observed at δ 8.0~8.2 ppm. The signal of the C–H protons in benzene were clearly observed at δ 7.0~8.0 ppm. The signal of the C–H protons in piperidine were observed at δ 1.0~5.0 ppm. Detailed 1H-NMR and 13C-NMR spectroscopy characteristics for the compounds are given in the Supporting Information (Figures S3–S6, S8–S53). For further confirmation, DEPT-135 analysis was performed (Figure S7). The signals in the negative direction relative to the original 13C-NMR spectrum confirm the existence of CH2 groups in the compounds.
2.2. Fungicidal Activities
The title compounds were evaluated for their fungicidal activities against six plant pathogenic fungi (Pythium aphanidermatum, Rhizoctonia solani, Valsa mali, Botrytis cinerea, Alternaria solani, and Gaeu-mannomyces graminsis) at the concentration of 50 mg/L as described in Section 3.3. The results were reported in Table 1. Fluopicolide, a commercial fungicide, was used as a positive control. Data listed in Table 1 showed that all the title compounds showed obvious fungicidal activities against all the six tested pathogenic fungi. Some compounds displayed satisfied activities, with inhibition rates against Pythium aphanidermatum, Rhizoctonia solani, Valsa mali, and Gaeu-mannomyces graminsis higher than 85%. Notably, compound 3b was found to display improved fungicidal activities against Rhizoctonia solani and Valsa mali (93%, 86%, respectively) compared with that of Fluopicolide (43%, 63%, respectively). The inhibitory rates of compound 3b against Pythium aphanidermatum and Gaeu-mannomyces graminsis reached 99%, similar to fluopicolide (99% and 95%, respectively).

Table 1.
In vitro fungicidal activities of the title compounds.
EC50 value of some compounds against Pythium aphanidermatum, Rhizoctonia solani, Valsa mali, and Gaeu-mannomyces graminsis were further tested (Table 2). In general, for Rhizoctonia solani, most compounds showed good antifungal activities with EC50 values lower than 10 μg/mL, and compounds 3c (R1 = H, R2 = 4-OCH2Ph) and 3f (R1 = 3-CH3, R2 = 2,6-Cl2) showed the best activities among all the title compounds with the EC50 values of 2.2 μg/mL and 2.1 μg/mL, respectively; for Pythium aphanidermatum, compound 3b (R1 = H, R2 = 2-OH,5-Cl), and compound 3l (R1 = 3-OH, R2 = 2-OH,5-Cl) displayed satisfied activities. Especially, compound 3b showed excellent activity with the EC50 value of 1.6 μg/mL, which is close to fluopicolide (EC50 = 1.0 μg/mL), a commercial fungicide to control Pythium aphanidermatum pathogens in the market. Structure-activity relationship study showed that: compounds (3b, 3g, 3i, and 3v) with 2-OH,5-Cl substituents on phenyl displayed better activities than those compounds with other substituents. In particular, the compound 3b containing unsubstituted piperidine displayed the most potent fungicidal activities against Rhizoctonia solani, Valsa mali, Gaeu-mannomyces graminsis, and Pythium aphanidermatum, with EC50 values of 9.6, 2.3, 9.3, and 1.6 μg/mL, respectively. Interestingly, compound 3z (Figure 4) without piperidine, although with 2-OH, 5-Cl substituents, exhibited much lower fungicidal activities than compound 3b. This indicated that the piperidine may play an important role on fungicidal activity.

Table 2.
The EC50 value of some title compounds.

Figure 4.
The chemical structure of compounds 3b and 3z.
3. Materials and Methods
3.1. General Information
Melting points of all compounds were determined on an X-4 binocular microscope (Fukai Instrument Co., Beijing, China) without calibration. NMR spectra were recorded on Bruker AM-300 (300 MHz) spectrometer with CDCl3 or DMSO-d6 as the solvent and TMS as the internal standard. Chemical shifts are reported in δ (parts per million) values. Elemental analysis was carried out on a Vario EL III elemental analyzer. High-resolution mass spectra were determined under electron impact (150 eV) conditions using a Bruker APEX IV instrument (Bruker Daltonics, Billerica, MA, USA). All the reagents, substituted piperidines, and benzaldehydes were obtained commercially and used without further purification.
3.2. Synthesis of Benzaldehyde Thiosemicarbazide Analogues 3a–3y
3.2.1. General Procedure for the Preparation of Intermediates 1
Intermediate 1 was synthesized using the published method [28]. A three-necked round bottom flask was charged with 22 mmol N,N′-thiocarbonyldiimidazole in 30 mL CH2Cl2. To the reaction flask 20 mmol substituted piperidine was added, and the reaction mixture was stirred at room temperature for 3 h. The mixture was washed with 100 mL water (3×). The organic layer was dried over sodium sulfate and the solvent was removed under reduced pressure. The resulting intermediate 1 was directly used in the next step without further purification. Intermediate 1a: yield 95%, yellow solid, m.p. = 85.0~86.0 °C; 1H-NMR (300 MHz, DMSO, Figure S1) δ 7.98 (t, J = 1.1 Hz, 1H, imidazole-H), 7.45 (t, J = 1.4 Hz, 1H, imidazole-H), 7.05~6.96 (m, 1H, imidazole-H), 4.49~3.27 (m, 4H, piperidine-H), 1.64 (m, 6H, piperidine-H).
3.2.2. General Procedure for the Preparation of Intermediates 2
On the basis of reported method [28], a three-necked round bottom flask was charged with 19 mmol intermediate 1 in 20 mL anhydrous ethanol. The reaction flask was charged with 19 mmol hydrazine hydrate (80%) via the addition funnel, which was a suspension in 10 mL anhydrous ethanol. The reaction mixture was stirred at room temperature for overnight. After intermediate 1 was completely consumed, the solvent ethanol was evaporated under reduced pressure, and the mixture was filtered and washed with cold ethanol. The resulting intermediate 2 was directly used in the next step without further purification. Intermediate 2a: yield 76%, white solid, m.p. = 93.0~94.0 °C; 1H-NMR (300 MHz, DMSO, Figure S2): 8.87 (s, 1H, NH), 4.66 (s, 2H, NH2), 3.70~3.61 (m, 4H, piperidine-H), 1.54 (m, 2H, piperidine-H), 1.42 (m, 4H, piperidine-H).
3.2.3. General Procedure of Benzaldehyde Thiosemicarbazide Analogues 3a–3t
The intermediate 2 (4.7 mmol) was added to a solution of corresponding substituted benzaldehyde (5 mmol) in 15 mL ethanol. Then three drops of glacial acetic acid were added to the reaction mixture as a catalyst. The mixture was stirred at room temperature for 10 min, corresponding precipitate was formed and then reaction 5 h at room temperature. The solid was filtered off and washed with ethanol to give the title compounds 3a–3t. The yields, physicochemical properties and structural characterization data of 3a–3t are as follows:
(E)-N′′-(2,6-Dichlorobenzylidene)piperidine-1-carbothiohydrazide (3a). White solid, m.p. 153.0~154.0 °C, yield 70%. IR (KBr), ν/cm−1: 3188, 1601, 1580, 1236, 777; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.69~1.44 (m, 6H, Piperidine-H), 4.01~3.73 (m, 4H, Piperidine-H), 7.43 (m, 3H, Ar-H), 8.34 (s, 1H, CH=N), 11.22 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.43, 138.44, 133.91, 130.92, 130.43, 129.29, 51.55, 25.90, 23.96; Anal. calcd. for C13H15Cl2N3S: C 49.37, H 4.78, N 13.29; found C 49.21, H 4.93, N 13.11.
(E)-N′-(5-Chloro-2-hydroxybenzylidene)piperidine-1-carbothiohydrazide (3b). Yellow solid, m.p. 174.0~175.0 °C, yield 52%. IR (KBr), ν/cm−1: 3352, 3003, 1614, 1535, 244, 829; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.59 (m, 6H, Piperidine-H), 3.99~3.78 (m, 4H, Piperidine-H), 6.90 (d, J = 8.8 Hz, 1H, Ar-H), 7.26 (dd, J = 8.8, 2.6 Hz, 1H, Ar-H), 7.51 (d, J = 2.6 Hz, 1H, Ar-H), 8.39 (s, 1H, CH=N), 11.48 (s, 1H, NH), 11.68 (s, 1H, Ar-OH); 13C-NMR (75 MHz, DMSO): 178.88, 155.87, 143.99, 130.20, 128.64, 122.67, 120.40, 118.45, 49.91, 25.66, 23.96; Anal. calcd. for C13H16ClN3OS: C 52.43, H 5.42, N 14.11; found C 52.31, H 5.20, N 13.99.
(E)-N′-(4-(Benzyloxy)benzylidene)piperidine-1-carbothiohydrazide (3c). Yellow solid, m.p. 120.0~121.0 °C, yield 66%. IR (KBr), ν/cm−1: 3151, 3033, 1603, 1506, 1248, 835; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.61 (s, 6H, Piperidine-H), 3.83 (s, 4H, Piperidine-H), 5.13 (s, 2H, Ar–CH2–O–), 7.05 (m, 2H, Ar-H), 7.61~7.25 (m, 7H, Ar-H), 8.05 (s, 1H, CH=N), 10.87 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.25, 159.71, 143.22, 136.90, 128.57, 128.34, 128.02, 127.84, 127.37, 115.34, 69.49, 51.37, 25.92, 24.07; Anal. calcd. for C20H23N3OS: C 67.96, H 6.56, N 11.89; found C 68.07, H 6.58, N 11.72.
(E)-N′-(3,5-Difluorobenzylidene)piperidine-1-carbothiohydrazide (3d). Yellow solid, m.p. 113.0~114.7 °C, yield 78%. IR (KBr), ν/cm−1: 3148, 3057, 1623, 1602, 1495, 1248, 858; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.62 (s, 6H, Piperidine-H), 3.85 (m, 4H, Piperidine-H), 7.39~7.15 (m, 3H, Ar-H), 8.08 (s, 1H, CH=N), 11.21 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.37, 164.40, 161.14, 140.54, 138.53, 109.61, 109.27, 105.02, 104.68, 104.33, 51.30, 25.87, 24.00; Anal. calcd. for C13H15F2N3S: C 55.11, H 5.34, N 14.83; found C 54.89, H 5.10, N 14.93.
(E)-N′-(3-Fluorobenzylidene)piperidine-1-carbothiohydrazide (3e). White solid, m.p. 108.0~109.0 °C, yield 54%. IR (KBr), ν/cm−1: 3175, 3075, 1603, 1576, 1507, 1244, 7813; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.62 (s, 6H, Piperidine-H), 3.85 (m, 4H, Piperidine-H), 7.21 (m, 1H, Ar-H), 7.52~7.36 (m, 3H, Ar-H), 8.10 (s, 1H, CH=N), 11.09 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.37 (s), 164.17 (s), 160.94 (s), 141.79 (d), 137.25 (d), 131.00 (d), 123.23 (d), 116.51 (s), 116.23 (s), 112.70 (s), 112.41 (s), 51.34 (s), 25.90 (s), 24.03 (s); HRMS (ESI+) m/z calcd for C13H16FN3S, 266.1122 [M], found 266.1122.
(E)-N′-(2,6-Dichlorobenzylidene)-3-methylpiperidine-1-carbothiohydrazide (3f). White solid, m.p. 104.9~105.9 °C, yield 48%. IR (KBr), ν/cm−1: 3153, 3047, 1580, 1557, 1505, 1243, 782; 1H-NMR (DMSO-d6, 300 MHz) δ: 0.94 (t, J = 6.0 Hz, 3H, CH3), 1.30~1.11 (m, 1H, Piperidine-CH), 1.94~1.61 (m, 4H, Piperidine-H), 2.95~2.74 (m, 1H, Piperidine-H), 3.21~3.09( m, 1H, Piperidine-H), 4.84~4.58 (m,2H, Piperidine-H), 7.46~7.20 (m, 3H, Ar-H), 7.90 (s, 1H, CH=N), δ 8.81 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.43, 138.48, 133.90, 130.97, 130.49, 129.28, 57.45, 51.06, 32.45, 31.19, 25.08, 18.77; Anal. calcd. for C14H17Cl2N3S: C 50.91, H 5.19, N 12.72; found C 51.05, H 5.36, N 12.93.
(E)-N′-(5-Chloro-2-hydroxybenzylidene)-3-methylpiperidine-1-carbothiohydrazide (3g). Yellow solid, m.p. 187.0~188.0 °C, yield 88%. IR (KBr), ν/cm−1: 3692, 1616, 1539, 1490, 1248, 837; 1H-NMR (DMSO-d6, 300 MHz) δ: 0.97 (d, J = 6.6 Hz, 3H, CH3), 1.33~1.14 (m, 1H, Piperidine-CH), 1.99~1.58 (m, 4H, Piperidine-H), 2.83 (m, 1H, Piperidine-H), 3.27~3.07 (m, 1H, Piperidine-H), 4.52 (m, 2H, Piperidine-H), 6.93 (m, 1H, Ar-H), 7.27~7.13 (m, 2H, Ar-H), 7.86 (s, 1H, CH=N), 8.62 (s, 1H, NH),10.49 (s, 1H, Ar-OH); 13C-NMR (75 MHz, DMSO): 178.88, 155.86, 143.91, 130.22, 128.57, 122.68, 120.42, 118.45, 55.85, 49.45, 32.48, 31.12, 24.97, 18.78; Anal. calcd. for C14H18ClN3OS: C 53.93, H 5.82, N 13.48; found C 53.80, H 5.71, N 13.39.
(E)-N′-(4-(Benzyloxy)benzylidene)-3-methylpiperidine-1-carbothiohydrazide (3h). Yellow solid, m.p. 122.0~123.4 °C, yield 60%. IR (KBr), ν/cm−1: 3160, 3032, 1606, 1507, 1243, 827; 1H-NMR (DMSO-d6, 300 MHz) δ: 0.86 (d, J = 6.6 Hz, 3H, CH3), 1.17 (m, 1H, Piperidine-H), 1.58~1.42 (m, 1H, Piperidine-H), 1.74~1.59 (m, 2H, Piperidine-H), 1.79 (m, 1H, Piperidine-H), 2.76 (m, 1H, Piperidine-H), 3.04 (m, 1H, Piperidine-H), 4.66~4.37 (m, 2H, Piperidine-H), 5.13 (s, 2H, Ar-CH2-O), 7.06 (m, 2H, Ar-H), 7.59~7.28 (m, 7H, Ar-H), 8.05 (s, 1H, CH=N), 10.88 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.13, 159.72, 143.28, 136.90, 128.57, 128.31, 128.02, 127.84, 127.37, 115.34, 69.49, 57.35, 50.88, 32.59, 31.28, 25.21, 18.81; HRMS (ESI+) m/z calcd for C21H25N3OS, 368.1791 (M), found 368.1794.
(E)-N′-(3,5-Difluorobenzylidene)-3-methylpiperidine-1-carbothiohydrazide (3i). Yellow solid, m.p. 111.0~112.0 °C, yield 22%. IR (KBr), ν/cm−1: 3155, 3096, 1619, 1507, 1247, 852; 1H-NMR (DMSO-d6, 300 MHz) δ: 0.87 (d, J = 6.6 Hz, 3H, CH3), 1.28~1.10 (m, 1H, Piperidine-H), 1.79~1.50 (m, 4H, Piperidine-H), 3.15~2.80 (m, 2H, Piperidine-H), 4.51 (m, 2H, Piperidine-H), 7.38~7.17 (m, 3H, Ar-H), 8.08 (s, 1H, CH=N), 11.23 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.26 (s), 164.40 (d), 161.14 (d), 140.53 (s), 138.59 (t), 109.41 (d), 104.6 (t), 57.24 (s), 50.85 (s), 32.52 (s), 31.30 (s), 25.17 (s), 18.70 (s); Anal. calcd. for C14H17F2N3OS: C 56.55, H 5.76, N 14.13; found C 56.69, H 5.70, N 13.97.°C
(E)-N′-(3-Fluorobenzylidene)-3-methylpiperidine-1-carbothiohydrazide (3j). Yellow solid, m.p. 81.0~82.0 °C, yield 21%. IR (KBr), ν/cm−1: 3155, 3096, 1619, 1507, 1247, 852; 1H-NMR (DMSO-d6, 300 MHz) δ: 0.87 (d, J = 6.5 Hz, 3H, CH3), 1.18 (m, 1H, Piperidine-H), 1.88~1.41 (m, 4H, Piperidine-H), 3.08~2.70 (m, 2H, Piperidine-H), 4.52 (m, 2H, Piperidine-H), 7.52~7.15 (m, 4H, Ar-H), 8.11 (s, 1H, CH=N), 11.10 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.28 (s), 164.18 (s), 160.95 (s), 141.81 (d), 137.26 (d), 131.00 (d), 123.23 (d), 116.52 (s), 116.23 (s), 112.63 (s), 112.33 (s), 57.30 (s), 50.89 (s), 32.54 (s), 31.30 (s), 25.19 (s), 18.74 (s); Anal. calcd. for C14H18FN3S: C 60.19, H 6.49, N 15.04; found C 60.03, H 6.20, N 14.93.
(E)-N′-(2,6-Dichlorobenzylidene)-3-hydroxypiperidine-1-carbothiohydrazide (3k). White solid, m.p. 164.8~165.9 °C, yield 37%. IR (KBr), ν/cm−1: 3329, 3217, 3049, 1581, 1544, 1511, 1252, 774; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.41 (m, 2H, Piperidine-H), 1.99~1.61 (m, 2H, Piperidine-H), 3.09 (m, 1H, Piperidine-H), 3.52~3.26 (m, 2H, Piperidine-H), 4.38 (m, 2H, Piperidine-H), 4.92 (d, J = 4.4 Hz, 1H, Piperidine-OH), 7.44 (m, 3H, Ar-H), 8.36 (s, 1H, CH=N), 11.26 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.96, 138.67, 133.94, 130.94, 130.36, 129.30, 65.43, 57.15, 50.59, 33.01, 22.94; Anal. calcd. for C13H15Cl2N3OS: C 47.00, H 4.55, N 12.65; found C 47.02, H 4.65, N 12.43.
(E)-N′-(5-Chloro-2-hydroxybenzylidene)-3-hydroxypiperidine-1-carbothiohydrazide (3l). White solid, m.p. 185.8~186.5 °C, yield 27%. IR (KBr), ν/cm−1: 3215, 3007, 1618, 1600, 1563, 1511, 1249, 817; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.43 (m, 2H, Piperidine-H), 1.81 (m, 2H, Piperidine-H), 3.16 (m, 1H, Piperidine-H), 3.53 (s, 2H, Piperidine-H), 4.23 (m, 1H, Piperidine-H), 4.44 (m, 1H, Piperidine-H), 4.98 (s, 1H, Piperidine-OH), 6.90 (d, J = 8.7 Hz, 1H, Ar-H), 7.26 (dd, J = 8.7, 2.5 Hz, 1H, Ar-H), 7.50 (d, J = 2.5 Hz, 1H, Ar-H), 8.39 (s, 1H, CH=N), 11.50 (s, 1H, NH), 11.68 (s, 1H, Ar-OH); 13C-NMR (75 MHz, DMSO): 179.46, 155.87, 144.10, 130.24, 128.70, 122.68, 120.35, 118.45, 65.24, 55.53, 49.10, 32.95, 22.66; Anal. calcd. for C13H16ClN3O2S: C 49.76, H 5.14, N 13.39; found C 50.01, H 5.31, N 13.34.
(E)-N′-(4-(Benzyloxy)benzylidene)-3-hydroxypiperidine-1-carbothiohydrazide (3m). Yellow solid, m.p. 152.6~153.7 °C, yield 46%. IR (KBr), ν/cm−1: 3315, 3177, 3008, 1602, 1511, 1259, 832; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.43 (m, 2H, Piperidine-H), 1.82 (m, 2H, Piperidine-H), 3.03 (m, 1H, Piperidine-H), 3.26~3.16 (m, 1H, Piperidine-H), 3.55 (m, 1H, Piperidine-H), 4.38 (m, 2H, Piperidine-H), 4.96 (d, J = 4.3 Hz, 1H, Piperidine-OH), 5.18 (s, 2H, Ar-CH2-O), 7.61~7.01 (m, 9H, Ar-H), 8.05 (s, 1H, CH=N), 10.92 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.61, 159.74, 143.39, 136.91, 128.58, 128.43, 128.03, 127.84, 127.32, 115.34, 69.49, 65.59, 57.07, 50.52, 33.19, 23.11; Anal. calcd. for C20H23N3O2S: C 65.02, H 6.27, N 11.37; found C 64.97, H 6.37, N 11.09.
(E)-N′-(3,5-Difluorobenzylidene)-3-hydroxypiperidine-1-carbothiohydrazide (3n). Yellow solid, m.p. 145.8~146.6 °C, yield 23%. IR (KBr), ν/cm−1: 3254, 3090, 3026, 1605, 1548, 1236, 851; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.44 (m, 2H, Piperidine-H), 1.88 (m, 2H, Piperidine-H), 3.10 (m, 1H, Piperidine-H), 3.26 (s, 1H, Piperidine-H), 3.56 (d, J = 4.1 Hz, 1H, Piperidine-H), 4.33 (m, 2H, Piperidine-H), 4.95 (d, J = 4.3 Hz, 1H, Piperidine-OH), 7.51~6.99 (m, 3H, Ar-H), 8.08 (s, 1H, CH=N), 11.25 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.79 (s), 164.39 (d), 161.13 (d), 140.66 (s), 138.53 (t), 109.50 (q), 104.6 (t), 65.55 (s), 56.93 (s), 50.52 (s), 33.03 (s), 22.93 (s); Anal. calcd. for C13H15F2N3OS: C 52.16, H 5.05, N 14.04; found C 52.20, H 5.13, N 13.98.
(E)-N′-(3-Fluorobenzylidene)-3-hydroxypiperidine-1-carbothiohydrazide (3o). Yellow solid, m.p. 127.0~128.0 °C, yield 34%. IR (KBr), ν/cm−1: 3064, 1605, 1558, 795; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.62~1.27 (m, 2H, Piperidine-H), 1.83 (m, 2H, Piperidine-H), 3.09 (m, 1H, Piperidine-H), 3.56~3.26 (m, 2H, Piperidine-H), 4.36 (m, 2H, Piperidine-H), 4.95 (d, J = 3.9 Hz, 1H, Piperidine-OH), 7.30~7.12 (m, 1H, Ar-H), 7.55~7.35 (m, 3H, Ar-H), 8.11 (s, 1H, CH=N), 11.13 (s, 1H, NH); 13C-NMR (75 MHz, DMSO): 180.79 (s), 164.18 (s), 160.94 (s), 141.95 (d), 137.20 (d), 130.99 (d), 123.33 (d), 116.56 (s), 116.27 (s), 112.76 (s), 112.46 (s), 65.56 (s), 56.99 (s), 50.53 (s), 33.09 (s), 22.99 (s); HRMS (ESI+) m/z calcd for C13H16FN3OS, 282.1071 [M], found 282.1069.
Ethyl (E)-1-(2-(2,6-dichlorobenzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylate (3p). White solid, m.p. 122.3~123.4 °C, yield 75%. IR (KBr), ν/cm−1: 3166, 3046, 1729, 1667, 1582, 1270; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.17 (t, J = 7.1 Hz, 3H, CH3), 1.70~1.49 (m, 2H, Piperidine-H), 1.87 (m, 2H, Piperidine-H), 2.76~2.59 (m, 1H, Piperidine-H), 3.30~3.20 (m, 2H, Piperidine-H), 4.06 (q, J = 7.1 Hz, 2H, CH2CH3), 4.55 (m, 2H, Piperidine-H), 7.40 (m, 1H, Ar-H), 7.54 (m, 2H, Ar-H), 8.36 (s, 1H, CH=N), 11.33 (s, 1H, NH); 13C-NMR (101 MHz, DMSO): 181.18, 174.25, 139.16, 134.26, 131.38, 130.67, 129.67, 60.47, 50.04, 28.46, 14.53; Anal. calcd. for C16H19Cl2N3O2S: C 49.49, H 4.93, N 10.82; found C 49.20, H 4.72, N 10.56.
Ethyl (E)-1-(2-(5-chloro-2-hydroxybenzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylate (3q). Yellow solid, m.p. 184.6~185.4 °C, yield 88%. IR (KBr), ν/cm−1: 3135, 3039, 1731, 1616, 1548, 1266, 822; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.18 (t, J = 7.1 Hz, 3H, CH3), 1.58 (m, 2H, Piperidine-H), 1.91 (m, 2H, Piperidine-H), 2.82~2.59 (m, 1H, Piperidine-H), 3.28 (m, 2H, Piperidine-H), 4.08 (q, J = 7.1 Hz, 2H, CH2CH3), 4.56 (m, 2H, Piperidine-H), 6.91 (d, J = 8.8 Hz, 1H, Ar-H), 7.27 (dd, J = 8.8, 2.7 Hz, 1H, Ar-H), 7.53 (d, J = 2.7 Hz, 1H, Ar-H), 8.40 (s, 1H, CH=N), 11.54 (s, 1H, NH), 11.63 (s, 1H, Ar-OH); 13C-NMR (101 MHz, DMSO): 179.64, 174.17, 156.22, 144.55, 130.66, 128.94, 123.06, 120.72, 118.80, 60.51, 48.45, 28.20, 14.54; Anal. calcd. for C16H20ClN3O3S: C 51.96, H 5.45, N 11.36; found C 52.20, H 5.54, N 11.43.
Ethyl (E)-1-(2-(4-(benzyloxy)benzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylate (3r). Yellow solid, m.p. 118.8~119.6 °C, yield 91%. IR (KBr), ν/cm−1: 3167, 3062, 1731, 1599, 1504, 1242, 829; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.19 (t, J = 7.1 Hz, 3H, CH3), 1.63 (m, 2H, Piperidine-H), 1.90 (m, 2H, Piperidine-H), 2.78~2.58 (m, 1H, Piperidine-H), 3.25 (m, 2H, Piperidine-H), 4.08 (q, J = 7.1 Hz, 2H, CH2CH3), 4.51 (m, 2H, Piperidine-H), 5.14 (s, 2H, Ar-CH2-O), 7.06 (d, J = 8.8 Hz, 2H, Ar-H), 7.49~7.27 (m, 5H, Ar-H), 7.55 (d, J = 8.8 Hz, 2H, Ar-H), 8.06 (s, 1H, CH=N),10.99 (s, 1H, NH); 13C-NMR (101 MHz, DMSO): 180.89, 174.33, 160.14, 143.99, 137.24, 128.92, 128.75, 128.37, 128.19, 127.59, 115.70, 69.83, 60.48, 49.80, 28.45, 14.55; Anal. calcd. for C23H27N3O3S: C 64.92, H 6.40, N 9.87; found C 64.99, H 6.34, N 9.88.
Ethyl (E)-1-(2-(3,5-difluorobenzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylate (3s). White solid, m.p. 127.2~128.3 °C, yield 89%. IR (KBr), ν/cm−1: 3139, 3059, 1728, 1609, 1539, 1515, 1273, 859; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.18 (t, J = 7.1 Hz, 3H, CH3), 1.63 (m, 2H, Piperidine-H), 1.92 (m, 2H, Piperidine-H), 2.88~2.58 (m, 1H, Piperidine-H), 3.26 (s, 2H, Piperidine-H), 4.08 (q, J = 7.1 Hz, 2H, CH2CH3), 4.50 (m, 2H, Piperidine-H), 7.49~7.03 (m, 3H, Ar-H), 8.09 (s, 1H, CH=N), 11.32 (s, 1H, NH); 13C-NMR (101 MHz, DMSO): 181.11 (s), 174.23 (s), 164.33 (d), 161.89 (d), 141.24 (s), 138.83 (t), 110.18~109.55 (q), 105.13 (t), 60.48 (s), 49.75 (s), 28.41 (s), 14.50 (s); Anal. calcd. for C16H19F2N3O2S: C 54.07, H 5.39, N 11.82; found C 54.35, H 5.34, N 11.81.
Ethyl (E)-1-(2-(3-fluorobenzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylate (3t). White solid, m.p. 130.0~131.0 °C, yield 78%. IR (KBr), ν/cm−1: 3139, 3055, 1728, 1608, 1515, 782; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.18 (t, J = 7.1 Hz, 3H, CH3),1.71~1.53 (m, 2H, Piperidine-H), 1.91 (m, 2H, Piperidine-H), 2.69 (m, 1H, Piperidine-H), 3.3 (m, 2H, Piperidine-H), 4.08 (q, J = 7.1 Hz, 2H, CH2CH3), 4.50 (m, 2H, Piperidine-H), 7.29~7.17 (m, 1H, Ar-H), 7.60~7.33 (m, 3H, Ar-H), 8.12 (s, 1H, CH=N), 11.20 (s, 1H, NH); 13C-NMR (101 MHz, DMSO): 181.09 (s), 174.26 (s), 164.11 (s), 161.69 (s), 142.54 (d), 137.49 (d), 131.37 (d), 123.66 (d), 116.80 (d), 112.92 (d), 60.48 (s), 49.78 (s), 28.43 (s), 14.52 (s); Anal. calcd. for C16H20F2N3O2S: C 56.96, H 5.97, N 12.45; found C 57.23, H 5.99, N 12.62.
3.2.4. General Procedure of Benzaldehyde Thiosemicarbazide Analoges 3u–3y
A three-necked round bottom flask was charged with compounds 3p–3t (8 mmol), 30 mL ethanol aqueous solution (Vethanol:Vwater = 2:1) was then added. After 16 mL 1.5 mol/L NaOH aqueous solution was added, the reaction mixture was stirred at room temperature for 2 h. TLC monitored the progress of the reaction, and after the ester (3p–3t) reaction completely, the reaction mixture was cooled to 0 °C, and concentrated HCl was added slowly until PH = 1. The solid was filtered off and washed with water to give the title compounds 3u–3y.
(E)-1-(2-(2,6-Dichlorobenzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylic acid (3u). White solid, m.p. 169.9~170.6 °C, yield 99.8%. IR (KBr), ν/cm−1: 3195, 3013, 1693, 1608, 1512, 778; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.57 (m, 2H, Piperidine-H), 1.86 (m, 2H, Piperidine-H), 2.56 (m, 1H, Piperidine-H), 3.25 (d, J = 11.0 Hz, 2H, Piperidine-H), 4.55 (m, 2H, Piperidine-H), 7.44 (m, 3H, Ar-H), 8.39 (s, 1H, CH=N), 11.37 (s, 1H, NH), 12.21 (s, 1H, COOH); 13C-NMR (101 MHz, DMSO): 181.07, 175.97, 139.30, 134.25, 131.33, 130.77, 129.64, 50.09, 40.35, 28.56; HRMS (ESI+) m/z calcd. for C14H15Cl2N3O2S, 360.0335 (M); found 360.0331.
(E)-1-(2-(5-Chloro-2-hydroxybenzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylic acid (3v). Yellow solid, m.p. 197.4~198.1 °C, yield 99.3%. IR (KBr), ν/cm−1: 3471, 3352, 3289, 1692, 1610, 1537, 1261, 834; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.57 (m, 2H, Piperidine-H), 1.90 (m, 2H, Piperidine-H), 2.69~2.53 (m, 1H, Piperidine-H), 3.28 (m, 2H, Piperidine-H), 4.56 (m, 2H, Piperidine-H), 6.91 (d, J = 8.8 Hz, 1H, Ar-H), 7.27 (dd, J = 8.8, 2.7 Hz, 1H, Ar-H), 7.52 (d, J = 2.7 Hz, 1H, Ar-H), 8.42 (s, 1H, CH=N), 11.66 (s, 3H, NH, OH, COOH); 13C-NMR (101 MHz, DMSO): 179.58, 175.90, 156.24, 144.62, 130.61, 128.98, 123.03, 120.70, 118.81, 48.59, 40.31, 28.29; Anal. calcd. for C16H16ClN3O3S: C 49.20, H 4.72, N 12.29; found C 48.91, H 4.79, N 12.05.
(E)-1-(2-(4-(Benzyloxy)benzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylic acid (3w). Yellow solid, m.p. 165.8~166.8 °C, yield 96%. IR (KBr), ν/cm−1: 3229, 3034, 1701, 1602, 1572, 1510, 1239, 840; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.61 (m, 2H, Piperidine-H), 1.90 (m, 2H, Piperidine-H), 2.58 (m, 1H, Piperidine-H), 3.24 (m, 2H, Piperidine-H), 4.51 (m, 2H, Piperidine-H), 5.14 (s, 2H, Ar-CH2-O), 7.07 (d, J = 8.6 Hz, 2H, Ar-H), 7.48~7.28 (m, 5H, Ar-H), 7.55 (d, J = 8.6 Hz, 2H, Ar-H), 8.07 (s, 1H, CH=N), 10.98 (s, 1H, NH), 12.28 (s, 1H, COOH); 13C-NMR (101 MHz, DMSO): 180.79, 176.23, 160.12, 143.94, 137.24, 128.92, 128.74, 128.38, 128.20, 127.62, 115.71, 69.83, 49.99, 40.63, 28.62; Anal. calcd. for C21H23N3O3S: C 63.46, H 5.83, N 10.57; found C 63.24, H 6.00, N 10.38.
(E)-1-(2-(3,5-Difluorobenzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylic acid (3x). White solid, m.p. 159.4~160.0 °C, yield 85%. IR (KBr), ν/cm−1: 3161, 3080, 1702, 1620, 1585, 1505, 1203, 860; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.60 (m, 2H, Piperidine-H), 1.90 (m, 2H, Piperidine-H), 2.59 (m, 1H, Piperidine-H), 3.30 (m, 2H, Piperidine-H), 4.49 (m, 2H, Piperidine-H), 7.51~7.09 (m, 3H, Ar-H), 8.09 (s, 1H, CH=N), 11.31 (s, 1H, NH), 12.30 (s, 1H, COOH); 13C-NMR (101 MHz, DMSO): 181.00 (s), 175.97 (s), 164.33 (d), 161.88 (d), 141.26 (s), 138.86 (t), 110.13~109.53 (q), 105.10 (t), 49.87 (s), 40.40 (s), 28.49 (s); Anal. calcd. for C14H15F2N3O2S: C 51.37, H 4.62, N 12.84; found C 51.10, H 4.42, N 12.59.
(E)-1-(2-(3-fluorobenzylidene)hydrazine-1-carbonothioyl)piperidine-4-carboxylic acid (3y). White solid, m.p. 165.2~165.9 °C, yield 87%. IR (KBr), ν/cm−1: 3155, 3080, 1708, 1608, 1515, 782; 1H-NMR (DMSO-d6, 300 MHz) δ: 1.61 (m, 2H, Piperidine-H), 1.90 (m, 2H, Piperidine-H), 2.59 (m, 1H, Piperidine-H), 3.35 (s, 2H, Piperidine-H), 4.50 (m, 2H, Piperidine-H), 7.67~7.04 (m, 4H, Ar-H), 8.11 (s, 1H, CH=N), 11.19 (s, 1H, NH), 12.32 (s, 1H, COOH); 13C-NMR (101 MHz, DMSO): 181.00 (s), 176.00 (s), 164.10 (s), 161.68 (s), 142.51 (d), 137.50 (d), 131.36 (d), 123.62 (d), 116.80 (d), 113.00 (d), 49.94 (s), 40.41 (s), 28.51 (s); Anal. calcd. for C14H16FN3O2S: C 54.36, H 5.21, N 13.58; found C 54.35, H 5.43, N 13.49.
3.3. Biological Assay
All the title compounds were evaluated for their in vitro fungicidal activities against six pathogenic fungi, using the mycelium growth rate method according to the literature [29]. Fungi tested in this article included Pythium aphanidermatum, Rhizoctonia solani, Valsa mali, Botrytis cinerea, Alternaria solani, and Gaeu-mannomyces graminsis. The tested compounds were weighted 10 mg and dissolved in 1 mL DMSO, prepared into 10,000 μg/mL concentration solution. Then it was mixed well with 199 mL PDA (potato dextrose agar). The medium containing compounds at a concentration of 50 mg/L for the initial screening was poured into sterilized Petri dishes (d = 90 cm), each group of three parallel tests. After the dishes were cooled, the mycelia disks of 7 mm diameter were inoculated in the center of the Petri dishes and incubated at 25 °C for 2–7 days. The DMSO without sample was used as blank control. The hypha diameter was measured by cross bracketing method. The commercial fungicides fluopicolide, azoxystrobin, and pyraclostrobin were used as positive controls. Fluopicolide is the main pesticide to control Pythium aphanidermatum pathogens on the market, and azoxystrobin and pyraclostrobin are the first and second largest fungicides on the market, respectively. The inhibition rate of the title compounds on the fungi was calculated by the following formula:
where C represents the average diameter of fungal growth on untreated PDA, and T represents the average diameter of fungi on treated PDA.
Inhibition rate (%) = [(C-T)/(C-7 mm)] × 100%
4. Conclusions
A series of novel benzaldehyde thiosemicarbazide derivatives containing heterocycle piperidine were designed and synthesized using the linking active substructure method. The in vitro bioassay results indicated that all the title compounds exhibited obvious antifungal activities against six pathogenic fungi, especially against Pythium aphanidermatum, Rhizoctonia solani, Valsa mali, and Gaeu-mannomyces graminsis. Compound 3b showed satisfying activity against four tested pathogenic fungi with EC50 values lower than 10 μg/mL. In particular, compound 3b, identified as the most potent inhibitor against Pythium aphanidermatum with an EC50 value of 1.6 μg/mL, showed similar activity to commercial Fluopicolide (EC50 = 1.0 μg/mL). The further in vivo trial of 3b is ongoing. SAR analysis shows that heterocycle piperidine plays an important role on fungicidal activity, and the results provide useful clues for further structural modification.
Supplementary Materials
The following are available online. Figures S1–S53: 1H-NMR spectra of compounds 1a, 2a, 3a–3y, 13C-NMR spectra of compounds 3a–3y, DEPT-135 spectra of compound 3b.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21672258 and No. 21472236) and the grant from the National Key Research and Development Plan (No. 2017YFD0200504).
Author Contributions
The current study is an outcome of constructive discussion with Y.L. and X.Y.; X.Z. carried out the synthesis, characterization, and antifungal bioassay experiments and involved in the drafting of the manuscript; P.L. involved in the synthesis of intermediates; T.S. and X.J. were partly involved in the antifungal bioassay; Y.L. and X.Y. were involved in the revising of the manuscript; and all authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wodnicka, A.; Huzar, E.; Krawczyk, M.; Kwiecien, H. Synthesis and antifungal activity of new salicylic acid derivatives. Pol. J. Chem. Technol. 2017, 19, 143–148. [Google Scholar] [CrossRef]
- Da Rocha Neto, A.C.; Luiz, C.; Maraschin, M.; Di Piero, R.M. Efficacy of salicylic acid to reduce Penicillium expansum inoculum and preserve apple fruits. Int. J. Food Microbiol. 2016, 221, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Stefan, B.; Arantxa, E.; Julia, K.; Carl, F.N. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Ji, H.B.; Wang, L.F. Advance in synthesis of natural benzaldehyde. Fine Chem. 2010, 6, 579–583. [Google Scholar]
- Eva, N.; Karel, W.; Jiri, K.; Karel, P.; Lenka, S. Design, synthesis, and biological evaluation of isothiosemicarbazones with antimycobacterial activity. Arch. Pharm. Chem. Life Sci. 2017, 350, e1700020. [Google Scholar]
- Cao, Y.H.; Zhang, Z.H.; Lin, F.K.; Lai, D.W.; Yao, Y.R.; Xie, B.Y.; Zhou, L.G. Secondary metabolites of endophytic fungus acremonium implicatum and their biological activities. Nat. Prod. Res. Dev. 2016, 28, 182–187. [Google Scholar]
- Maruthachalam, M.; Ganesan, A.; Gunasekaran, R.; Chinnasamy, J. Synthesis, characterization, DNA binding, DNA cleavage, antioxidant and in vitro cytotoxicity studies of ruthenium(II) complexes containing hydrazone ligands. J. Coord. Chem. 2016, 69, 3545–3559. [Google Scholar]
- Haraguchi, S.K.; Silva, A.A.; Vidotti, G.J.; dos Santos, P.V.; Garcia, F.P.; Pedroso, R.B.; Nakamura, C.V.; de Oliveira, C.M.; da Silva, C.C. Antitrypanasomal activity of novel benzaldehyde-thiosemicarbazone derivatives from kaurenoic acid. Molecules 2011, 16, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Augeri, D.J.; Bencivenga, A.F.; Blanden, A.; Carpizo, D.R.; Gilleran, J.A.; Kimball, S.D.; Loh, S.N.; Yu, X. Preparation of Thiosemicarbazone Derivatives Useful for Treatment of Cancer. Patent WO 2016123242, 4 August 2016. [Google Scholar]
- Chibale, K.; Greenbaum, D.C.; McKerrow, J.H. Thiosemicarbazones and Other Compounds as Antiparasitic Compounds, Their Preparation, and Methods of Their Use. Patent WO 2005087211, 22 September 2005. [Google Scholar]
- Shi, D.Y.; Wang, L.J.; Guo, C.L.; Jiang, B.; Wang, S.Y.; Zhao, Y. Process for Preparation of Bromophenol Amino Thiourea Compounds, and Pharmaceutical Activity. Patent CN 106748939, 31 May 2017. [Google Scholar]
- Song, S.C.; Zhu, G.X.; Chen, Z.Y.; You, A.; Qi, J.; Liang, H.; Pan, W.L. Alkyl Substituted Benzaldehyde or Acetophenone Thiosemicarbazone Derivatives as Tyrosinase Inhibitors and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Aging and Skin Pigmentation. Patent CN 105439926, 30 March 2016. [Google Scholar]
- Song, S.C.; You, A.; Zhu, G.X.; Yi, Z.; Qi, J.; Chen, Z.Y.; Liang, H.; Pan, W.L. Aryl-Substituted Benzaldehyde or Acetophenone Thiosemicarbazone Derivative as Tyrosinase Inhibitor and Their Preparation. Patent CN 105294527, 3 February 2016. [Google Scholar]
- Tang, X.R.; Yang, J.; Gao, S.M.; Liu, H.; Gao, Y.; Li, W.Y.; Zeng, Y.; Xu, Z.H.; Zhang, Y.; Wang, L. Thiosemicarbazone Derivative, Its Preparation and Application in Controlling Plant Pathogenic Fungi. Patent CN 105384722, 9 March 2016. [Google Scholar]
- Liu, C.L.; Dong, X.W.; Zhang, Z.; Lei, J.; Chen, H.H.; Zhao, J.D. Semicarbazone Compounds as SOD1 Inhibitors and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Diseases Caused by Copper Metabolic Disorders. Patent CN 105017197, 4 November 2015. [Google Scholar]
- Wang, W.; Yao, J.; Cao, W.; Liu, X.D. Prepration of Hydrazinethioamide Compound from Aminothiourea, Hydroxybenzaldehyde and Aromatic Diamine. Patent CN 103709082, 9 April 2014. [Google Scholar]
- Wang, Z.; Dong, W.; Xu, Y.; Liang, P.; Yang, X.L. Synthesis of substituted benzylidene hydrazinecarbothioamide (hydrazinecarboxamide, nitrohydrazinecarboximidamide) and their inhibitory activity on tyrosinase of diamondback moth Plutella xylostella (L.). Nongyaoxue Xuebao 2010, 12, 264–268. [Google Scholar]
- Meena, P.; Nemaysh, V.; Khatri, M.; Manral, A.; Luthra, P.M.; Tiwari, M. Synthesis, biological evaluation and molecular docking study of novel piperidine and piperazine derivatives as multi-targeted agents to treat Alzheimer’s disease. Bioorg. Med. Chem. 2015, 23, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Zhang, X.; Xu, Y.; Xu, G.; Liu, X.; Yang, X.; Zhang, X.; Ling, Y. Synthesis and fungicidal activity of pyrazole derivatives containing 1,2,3,4-tetrahydroquinoline. Chem. Cent. J. 2016, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.N.; Zheng, M.Y.; Miao, F.X.; Yu, J.Q.; Li, S.H.; Li, F.N.; Qu, N. Synthesis and anti-hepatoma activities of N-(piperidine-4-yl)benzamide derivatives. Yingyong Huaxue 2016, 33, 661–667. [Google Scholar]
- Pivatto, M.; Baccini, L.R.; Sharma, A.; Nakabashi, M.; Danuello, A.; Viegas, C.J.; Garcia, C.R.S.; Bolzani, V.S. Antimalarial activity of piperidine alkaloids from Senna spectabilis and semisynthetic derivatives. J. Braz. Chem. Soc. 2014, 25, 1900–1906. [Google Scholar]
- Rajput, A.P.; Nagarale, D.V. Synthesis, characterization and antimicrobial study of piperidine-2,6-diones derivatives. Pharm. Chem. 2016, 8, 182–186. [Google Scholar]
- Li, F.Y.; Zhu, Y.J.; Fan, Z.J.; Xu, J.H.; Guo, X.F.; Zong, G.N.; Song, H.B.; Chen, L.; Song, Y.Q.; Qian, X.L. Synthesis, crystal structure and biological activity of 2-(1-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carbonyl)piperidine-4-yl)-N-isopropyl-1,3-thiazole-4-carboxamide. Chin. J. Struct. Chem. 2015, 34, 659–666. [Google Scholar]
- Shaw, S.A.; Balasubramanian, B.; Bonacorsi, S.; Cortes, J.C.; Cao, K.; Chen, B.C.; Dai, J.; Decicco, C.; Goswami, A.; Guo, Z.W. Synthesis of biologically active piperidine metabolites of clopidogrel: determination of structure and analyte development. J. Org. Chem. 2015, 80, 7019–7032. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, X.C.; Yu, M.C.; Xiao, L.; Zhang, X.J.; Sun, H.J.; Chen, H.; Pan, G.X.; Yan, Y.R.; Wang, S.C. Discovery, synthesis, biological evaluation and structure-based optimization of novel piperidine derivatives as acetylcholine-binding protein ligands. Acta Pharmacol. Sin. 2017, 38, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Pasteris, R.J.; Hanagan, M.A.; Bisaha, J.J.; Finkelstein, B.L.; Hoffman, L.E.; Gregory, V.; Andreassi, J.L.; Sweigard, J.A.; Klyashchitsky, B.A.; Henry, Y.T.; et al. Discovery of oxathiapiprolin, a new oomycete fungicide that targets an oxysterol binding protein. Bioorg. Med. Chem. 2016, 24, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, K.; Iwamoto, S.; Shigeta, Y.; Hirokawa, Y.; Oowada, S.; Nakamura, T.; Ishiwata, N. Preparation of Pyrazolone Compounds as Thrombopoietin Receptor Activators. U.S. Patent 2007142308, 13 December 2007. [Google Scholar]
- Du, S.; Tian, Z.; Yang, D.; Li, X.; Li, H.; Jia, C.; Che, C.; Wang, M.; Qin, Z. Synthesis, antifungal activity and structure-activity relationships of novel 3-(difluoromethyl)-1-methyl-1h-pyrazole-4-carboxylic acid amides. Molecules 2015, 20, 8395–8408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3a–3y are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).