Extraction of Fenugreek (Trigonella foenum-graceum L.) Seed Oil Using Subcritical Butane: Characterization and Process Optimization
Abstract
:1. Introduction
2. Results and Discussion
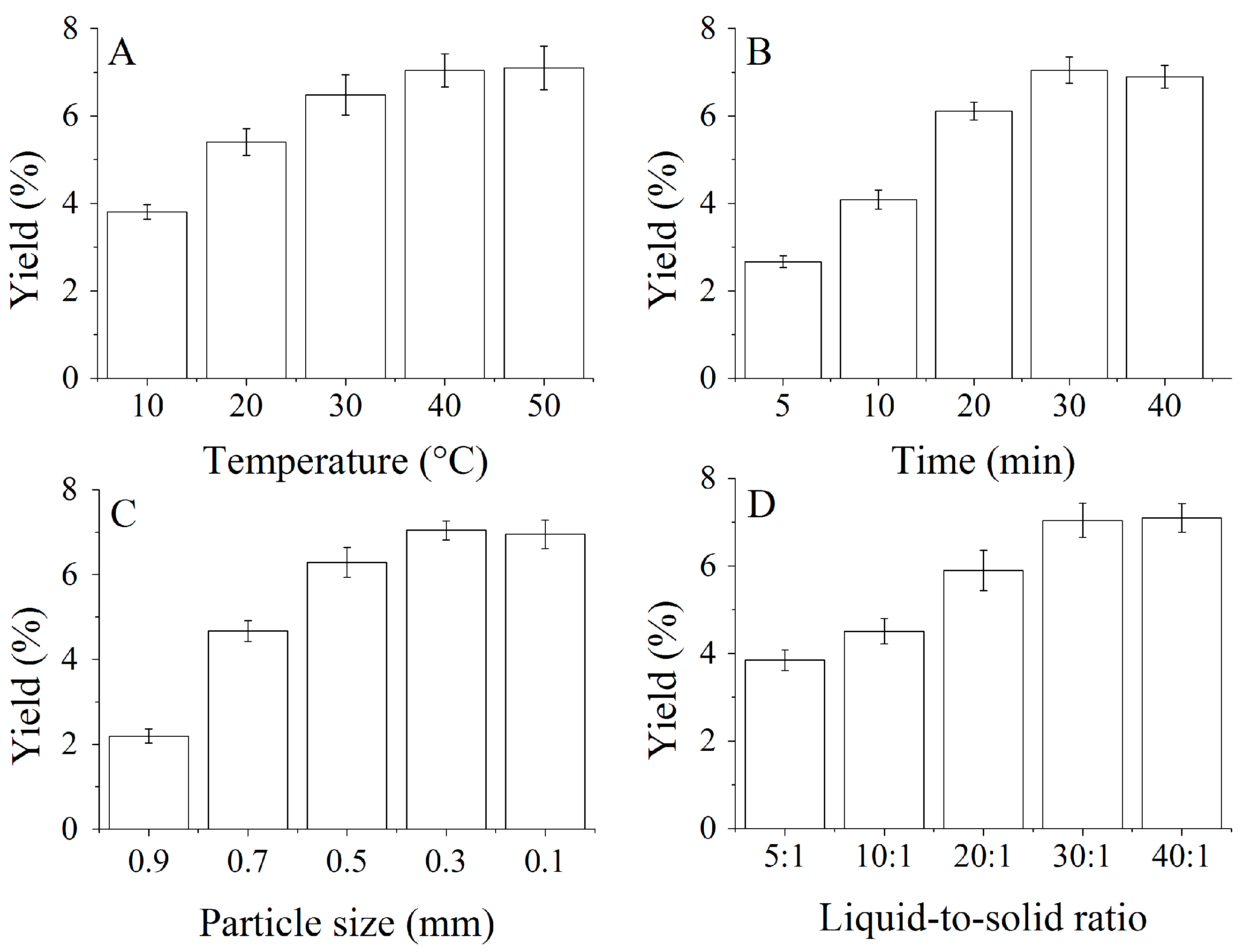
2.1. Effects of Single Factors
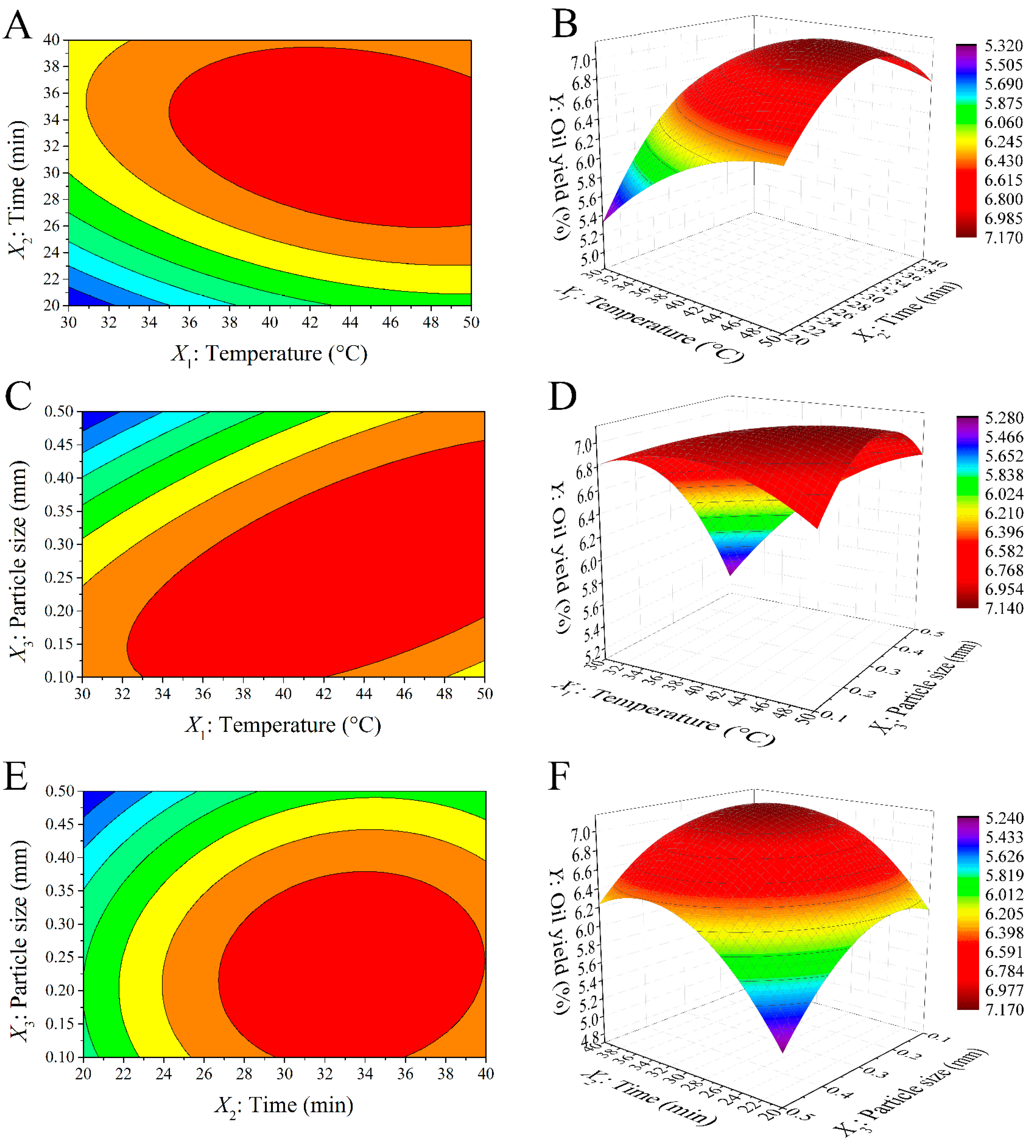
2.2. Response Surface Optimization of SBE
2.2.1. ANOVA Analysis and the Model Fitting
2.2.2. Response Surface Analysis
2.3. Optimization of Extraction Conditions
2.4. Comparison of SBE and ASE on Oil Yield and Fatty Acid Composition
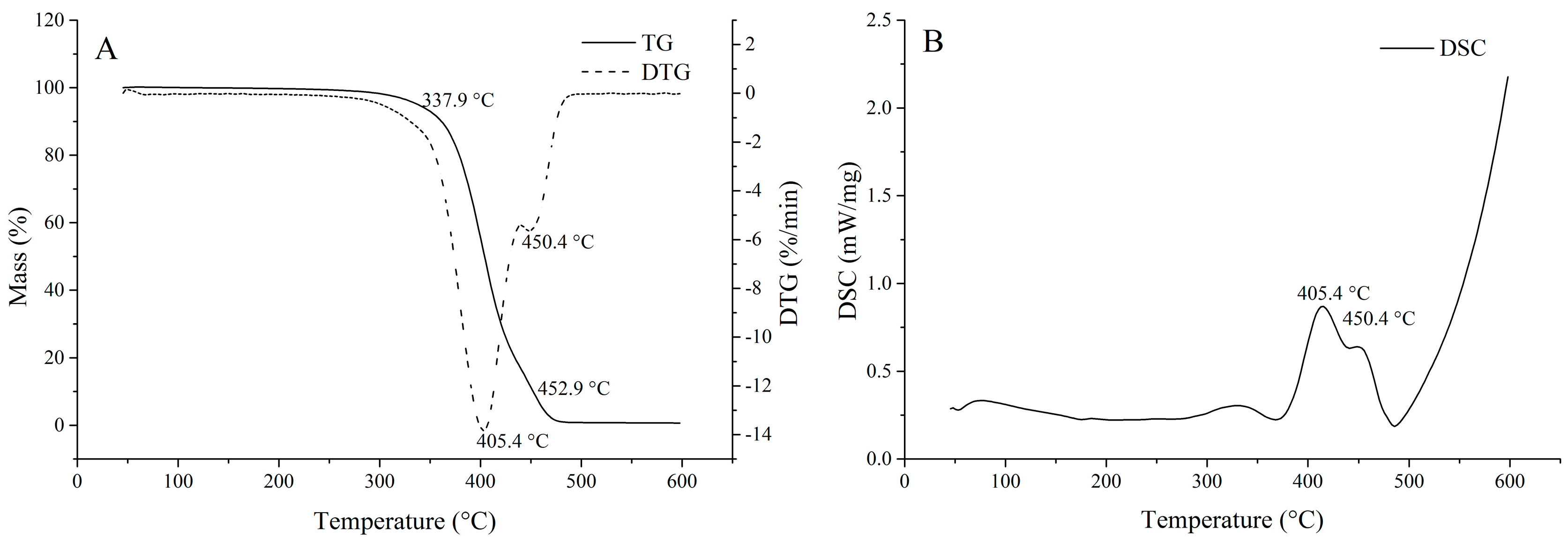
2.5. Physicochemical Characteristics of the Oil
3. Materials and Methods
3.1. Materials
3.2. Oil Extraction
3.3. Experimental Design
3.3.1. Single-Factor Experiments
3.3.2. Response Surface Methodology
3.4. Characterization of Seed Oil
3.5. Determination of Fatty Acid
3.6. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naidu, M.M.; Shyamala, B.N.; Naik, J.P.; Sulochanamma, G.; Srinivas, P. Chemical composition and antioxidant activity of husk and endosperm of fenugreek seeds. LWT Food Sci. Technol. 2011, 44, 451–456. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Ali, A.O.; Hemavathy, J. Lipid content and fatty acid composition of fenugreek (Trigonella foenum-graecum L.) seeds grown in Sudan. Int. J. Food Sci. Technol. 2008, 43, 380–382. [Google Scholar] [CrossRef]
- Lust, J.B. The Herb Book; Bantam Books: New York, NY, USA, 1986. [Google Scholar]
- El-Bahy, G. FTIR and Raman spectroscopic study of fenugreek (Trigonella foenum-graecum L.) seeds. J. Appl. Spectrosc. 2005, 72, 111–116. [Google Scholar] [CrossRef]
- Savitha, H.G.; Manohar, B. Studies on grinding and extraction of oil from fenugreek (Trigonella foenum-graecum) seed. Int. J. Food Eng. 2015, 11, 275–283. [Google Scholar]
- Prasad, R. Identification of High Seed Yielding and Stable Fenugreek Mutants. Masters’ Thesis, University of Lethbridge, Lethbridge, AB, Canada, 2014. [Google Scholar]
- Ren, X.F.; Zhu, W.J. Optimal conditions for extraction of oil from fenugreek (Trigonella foenum-graecum L.) by supercritical CO2 fluids (SFE-CO2). Adv. Mater. Res. 2011, 236–238, 2980–2983. [Google Scholar] [CrossRef]
- Schuette, H.A.; Cowley, M.A.; Vogel, H.A.; Mueller, M.M. Fenugreek seed oil. J. Am. Oil Chem. Soc. 1940, 17, 122. [Google Scholar] [CrossRef]
- Arivalagan, M.; Gangopadhyay, K.K.; Kumar, G. Determination of steroidal saponins and fixed oil content in fenugreek (Trigonella foenum-graecum) genotypes. Indian J. Pharm. Sci. 2013, 75, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Natolino, A.; Decorti, D. Effect of ultrasound pre-treatment of hemp (Cannabis sativa L.) seed on supercritical CO2 extraction of oil. J. Food Sci. Technol. 2015, 52, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, Y.; Zhang, R.; Ma, H.; Liu, B. Preparation and characterization of foxtail millet bran oil using subcritical propane and supercritical carbon dioxide extraction. J. Food Sci. Technol. 2015, 52, 3099–3104. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, K. Accelerated solvent extraction of lipids for determining the fatty acid composition of biological material. Anal. Chim. Acta 1998, 358, 69–77. [Google Scholar] [CrossRef]
- Peterson, J.; Carison, R.; Richter, B.; Knowles, D. Extraction of oil from oilseeds using accelerated solvent extraction (ASE). LCGC N. Am. 2009, 36. [Google Scholar]
- Santos, K.A.; Bariccatti, R.A.; Cardozo-Filho, L.; Schneider, R.; Palú, F.; da Silva, C.; da Silva, E.A. Extraction of crambe seed oil using subcritical propane: Kinetics, characterization and modeling. J. Supercrit. Fluids 2015, 104, 54–61. [Google Scholar] [CrossRef]
- Liu, Z.; Mei, L.; Wang, Q.; Shao, Y.; Tao, Y. Optimization of subcritical fluid extraction of seed oil from Nitraria tangutorum using response surface methodology. LWT Food Sci. Technol. 2014, 56, 168–174. [Google Scholar] [CrossRef]
- Li, J.; Sun, D.; Qian, L.; Liu, Y. Subcritical butane extraction of wheat germ oil and its deacidification by molecular distillation. Molecules 2016, 21, 1675. [Google Scholar] [CrossRef] [PubMed]
- Zanqui, A.B.; de Morais, D.R.; da Silva, C.M.; Santos, J.M.; Marques Gomes, S.T.; Visentainer, J.V.; Eberlin, M.N.; Cardozo-Filho, L.; Matsushita, M. Subcritical extraction of flaxseed oil with n-propane: Composition and purity. Food Chem. 2015, 188, 452–458. [Google Scholar] [PubMed]
- Li, H.Z.; Zhang, Z.J.; Hou, T.Y.; Li, X.J.; Chen, T. Optimization of ultrasound-assisted hexane extraction of perilla oil using response surface methodology. Ind. Crops Prod. 2015, 76, 18–24. [Google Scholar] [CrossRef]
- Liu, S.; Feng, Y.; Zhang, C.; Ji, H.; Hong, P.; Deng, C. Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J. Supercrit. Fluids 2009, 48, 9–14. [Google Scholar] [CrossRef]
- Ni, Q.; Gao, Q.; Yu, W.; Liu, X.; Xu, G.; Zhang, Y. Supercritical carbon dioxide extraction of oils from two Torreya grandis varieties seeds and their physicochemical and antioxidant properties. LWT Food Sci. Technol. 2015, 60, 1226–1234. [Google Scholar] [CrossRef]
- Amin, A.; Alkaabi, A.; Al-Falasi, S.; Daoud, S.A. Chemopreventive activities of Trigonella foenum-graecum (fenugreek) against breast cancer. Cell Biol. Int. 2005, 29, 687–694. [Google Scholar] [CrossRef] [PubMed]
- El Nasri, N.A.; El Tinay, A.H. Functional properties of fenugreek (Trigonella foenum graecum) protein concentrate. Food Chem. 2007, 103, 582–589. [Google Scholar] [CrossRef]
- Brummer, Y.; Cui, W.; Wang, Q. Extraction, purification and physicochemical characterization of fenugreek gum. Food Hydrocoll. 2003, 17, 229–236. [Google Scholar] [CrossRef]
- Hamden, K.; Keskes, H.; Belhaj, S.; Mnafgui, K.; Allouche, N. Inhibitory potential of omega-3 fatty and fenugreek essential oil on key enzymes of carbohydrate-digestion and hypertension in diabetes rats. Lipids Health Dis. 2011, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, R.; Mahmoudi, A.; Fouchet, M.; Dos Santos, M.; Kamissoko, F.; Nafti, A.; Cheikh, R.B.; Rega, B.; Camel, V. Characterisation of volatile compounds in Tunisian fenugreek seeds. Food Chem. 2009, 115, 1326–1336. [Google Scholar] [CrossRef]
- Gu, L.B.; Pang, H.L.; Lu, K.K.; Liu, H.M.; Wang, X.D.; Qin, G.Y. Process optimization and characterization of fragrant oil from red pepper (Capsicum annuum L.) seed extracted by subcritical butane extraction. J. Sci. Food Agric. 2016. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Chang, Z.; Duan, P.; Yan, W.; Xu, Y.; Lei, Z.; Miao, J.; Fan, Y. Hydrothermal liquefaction of Litsea cubeba seed to produce bio-oils. Bioresour. Technol. 2013, 149, 509–515. [Google Scholar]
- Rai, A.; Mohanty, B.; Bhargava, R. Supercritical extraction of sunflower oil: A central composite design for extraction variables. Food Chem. 2016, 192, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Duba, K.S.; Fiori, L. Supercritical CO2 extraction of grape seed oil: Effect of process parameters on the extraction kinetics. J. Supercrit. Fluids 2015, 98, 33–43. [Google Scholar] [CrossRef]
- Zermane, A.; Larkeche, O.; Meniai, A.H.; Crampon, C.; Badens, E. Optimization of essential oil supercritical extraction from Algerian Myrtus communis L. leaves using response surface methodology. J. Supercrit. Fluids 2014, 85, 89–94. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Škevin, D.; Valić, S.; Majetić, V.; Petričević, S.; Ljubenkov, I. The antioxidant capacity and oxidative stability of virgin olive oil enriched with phospholipids. Food Chem. 2008, 111, 121–126. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, P.; Xiao, Y.; Liu, Q. Optimization of supercritical carbon dioxide extraction, physicochemical and cytotoxicity properties of Gynostemma pentaphyllum seed oil: A potential source of conjugated linolenic acids. Sep. Purif. Technol. 2016, 159, 147–156. [Google Scholar] [CrossRef]
- Abdulkarim, S.; Long, K.; Lai, O.; Muhammad, S.; Ghazali, H. Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem. 2005, 93, 253–263. [Google Scholar] [CrossRef]
- Nehdi, I.A.; Sbihi, H.; Tan, C.P.; Zarrouk, H.; Khalil, M.I.; Al-Resayes, S.I. Characteristics, composition and thermal stability of Acacia senegal (L.) willd. seed oil. Ind. Crops Prod. 2012, 36, 54–58. [Google Scholar] [CrossRef]
- Fontanel, D. Unsaponifiable Matter in Plant Seed Oils; Springer: New York, NY, USA, 2013. [Google Scholar]
- Lee, J.W.; Lee, S.W.; Kim, M.K.; Rhee, C.; Kim, I.H.; Lee, K.W. Beneficial effect of the unsaponifiable matter from rice bran on oxidative stress in vitro compared with α-tocopherol. J. Sci. Food Agric. 2005, 85, 493–498. [Google Scholar] [CrossRef]
- Sathivel, S.; Yin, H.; Prinyawiwatkul, W.; King, J.M. Comparisons of chemical and physical properties of catfish oils prepared from different extracting processes. J. Food Sci. 2009, 74, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Santos, I.; Conceiçăo, M.; Porto, S.; Trindade, M.; Souza, A.; Prasad, S.; Fernandes, V.; Araújo, A. Thermoanalytical, kinetic and rheological parameters of commercial edible vegetable oils. J. Therm. Anal. Calorim. 2004, 75, 419–428. [Google Scholar] [CrossRef]
- Sample Availability: Fenugreek seed was purchased from a regional pharmacy, located at Zhengzhou, China, and the seed oils are available from the authors.

| Source 1 | Coefficient Estimate | Standard Error | Sum of Squares | DF | Mean Square | F-Value | p-Value 2 |
|---|---|---|---|---|---|---|---|
| Model | N/A | N/A | 6.41 | 9 | 0.71 | 26.29 | 0.0001 |
| Intercept | 7.04 | 0.074 | N/A | N/A | N/A | N/A | N/A |
| X1 | 0.31 | 0.058 | 0.77 | 1 | 0.77 | 28.34 | 0.0011 |
| X2 | 0.39 | 0.058 | 1.24 | 1 | 1.24 | 45.95 | 0.0003 |
| X3 | −0.33 | 0.058 | 0.88 | 1 | 0.88 | 32.46 | 0.0007 |
| X1X2 | −0.22 | 0.082 | 0.19 | 1 | 0.19 | 6.95 | 0.0336 |
| X1X3 | 0.43 | 0.082 | 0.76 | 1 | 0.76 | 27.90 | 0.0011 |
| X2X3 | 0.099 | 0.082 | 0.039 | 1 | 0.039 | 1.44 | 0.2692 |
| X12 | −0.26 | 0.080 | 0.28 | 1 | 0.28 | 10.25 | 0.0150 |
| X22 | −0.54 | 0.080 | 1.24 | 1 | 1.24 | 45.88 | 0.0003 |
| X32 | −0.43 | 0.080 | 0.77 | 1 | 0.77 | 28.56 | 0.0011 |
| Residual | N/A | N/A | 0.19 | 7 | 0.027 | N/A | N/A |
| Lack of Fit | N/A | N/A | 0.14 | 3 | 0.047 | 3.72 | 0.1183 |
| Pure Error | N/A | N/A | 0.050 | 4 | 0.012 | N/A | N/A |
| SD | 0.16 | N/A | R2 | 0.9713 | N/A | N/A | N/A |
| Mean | 6.47 | N/A | Adj. R2 | 0.9343 | N/A | N/A | N/A |
| CV (%) | 2.55 | N/A | Pred. R2 | 0.6497 | N/A | N/A | N/A |
| PRESS | 2.31 | N/A | Adeq. Precision | 14.233 | N/A | N/A | N/A |
| Fatty Acid | SBE 1 (%) | ASE 2 (%) |
|---|---|---|
| Myristic acid (C14:0) | 0.14 ± 0.01 | 0.14 ± 0.01 |
| Palmitic acid (C16:0) | 10.00 ± 0.16 | 9.94 ± 0.13 |
| Stearic acid (C18:0) | 4.71 ± 0.13 | 4.68 ± 0.20 |
| Oleic acid (C18:1) | 14.24 ± 0.08 | 14.40 ± 0.14 |
| Linoleic acid (C18:2) | 42.80 ± 0.11 | 42.71 ± 0.13 |
| Arachidic acid (C20:0) | 1.33 ± 0.03 | 1.33 ± 0.04 |
| Linolenic acid (C18:3) | 26.15 ± 0.20 | 26.03 ± 0.12 |
| Behenic acid (C22:0) | 0.63 ± 0.01 | 0.59 ± 0.03 |
| Erucyl alcohol (C22:1) | ND 3 | 0.20 ± 0.02 |
| Properties | Values | |
|---|---|---|
| Color | red units | 9.516 ± 0.133 |
| yellow units | 70.647 ± 1.612 | |
| blue units | 0.586 ± 0.049 | |
| Refractive index | 1.479 ± 0.233 | |
| Relative density | 0.922 ± 0.021 | |
| Acid value (mg/g oil) | 6.413 ± 0.196 | |
| Iodine value (g/100 g oil) | 148.564 ± 2.025 | |
| Saponification value (mg KOH/g oil) | 190.277 ± 2.391 | |
| Unsaponifiable matter (%) | 3.790 ± 0.215 | |
| Peroxide value (meq. O2/kg oil) | 0.627 ± 0.033 | |
| Induction time (h, 120 °C) | 2.850 ± 0.081 | |
| α-Tocopherol (mg·100 g−1) | 16.460 ± 0.667 | |
| RUN | Independent Variable | Oil Yield (%) | |||
|---|---|---|---|---|---|
| X1 (Temperature, °C) | X2 (Time, min) | X3 (Particle Size, mm) | Experimental | Predicted | |
| 1 | 50 | 30 | 0.1 | 6.75 ± 0.26 | 6.57 |
| 2 | 40 | 30 | 0.3 | 6.94 ± 0.31 | 7.04 |
| 3 | 40 | 20 | 0.5 | 5.33 ± 0.23 | 5.25 |
| 4 | 50 | 30 | 0.5 | 6.74 ± 0.12 | 6.77 |
| 5 | 40 | 30 | 0.3 | 6.93 ± 0.28 | 7.04 |
| 6 | 40 | 30 | 0.3 | 7.06 ± 0.36 | 7.04 |
| 7 | 40 | 30 | 0.3 | 7.12 ± 0.11 | 7.04 |
| 8 | 30 | 20 | 0.3 | 5.43 ± 0.09 | 5.32 |
| 9 | 50 | 20 | 0.3 | 6.33 ± 0.27 | 6.38 |
| 10 | 50 | 40 | 0.3 | 6.63 ± 0.30 | 6.73 |
| 11 | 40 | 30 | 0.3 | 7.18 ± 0.28 | 7.04 |
| 12 | 40 | 40 | 0.1 | 6.62 ± 0.17 | 6.70 |
| 13 | 30 | 30 | 0.5 | 5.10 ± 0.10 | 5.28 |
| 14 | 30 | 30 | 0.1 | 6.85 ± 0.25 | 6.82 |
| 15 | 30 | 40 | 0.3 | 6.59 ± 0.14 | 6.55 |
| 16 | 40 | 20 | 0.1 | 5.98 ± 0.33 | 6.11 |
| 17 | 40 | 40 | 0.5 | 6.37 ± 0.21 | 6.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.-B.; Liu, X.-N.; Liu, H.-M.; Pang, H.-L.; Qin, G.-Y. Extraction of Fenugreek (Trigonella foenum-graceum L.) Seed Oil Using Subcritical Butane: Characterization and Process Optimization. Molecules 2017, 22, 228. https://doi.org/10.3390/molecules22020228
Gu L-B, Liu X-N, Liu H-M, Pang H-L, Qin G-Y. Extraction of Fenugreek (Trigonella foenum-graceum L.) Seed Oil Using Subcritical Butane: Characterization and Process Optimization. Molecules. 2017; 22(2):228. https://doi.org/10.3390/molecules22020228
Chicago/Turabian StyleGu, Ling-Biao, Xiao-Ning Liu, Hua-Min Liu, Hui-Li Pang, and Guang-Yong Qin. 2017. "Extraction of Fenugreek (Trigonella foenum-graceum L.) Seed Oil Using Subcritical Butane: Characterization and Process Optimization" Molecules 22, no. 2: 228. https://doi.org/10.3390/molecules22020228
APA StyleGu, L.-B., Liu, X.-N., Liu, H.-M., Pang, H.-L., & Qin, G.-Y. (2017). Extraction of Fenugreek (Trigonella foenum-graceum L.) Seed Oil Using Subcritical Butane: Characterization and Process Optimization. Molecules, 22(2), 228. https://doi.org/10.3390/molecules22020228





