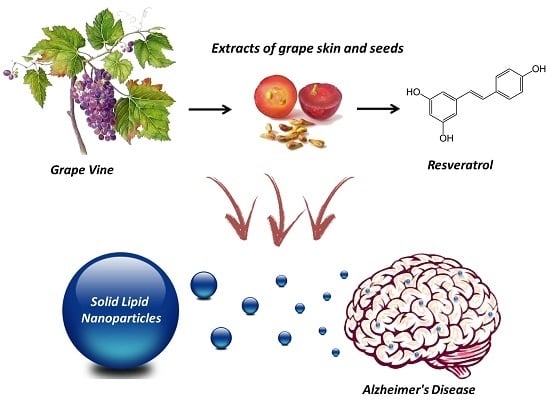
Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impact of Resveratrol and Extracts of Grape Skin and Seed on Aβ(1–42) Fibrillation
2.2. Solid Lipid Nanoparticles Formulation
2.3. Solid Lipid Nanoparticle Stability
2.4. Effect of the Loaded Solid Lipid Nanoparticles on Amyloid-β Aggregation
2.5. Conjugation of the Antibodies
2.6. Uptake and Transport Assays
3. Materials and Methods
3.1. Stock Solutions of Amyloid-β Peptide
3.2. Stock Solutions of Resveratrol, Extracts of Grape Seed and Skin
3.3. Thioflavin T Binding Assay
3.4. Transmission Electron Microscopy
3.5. Solid Lipid Nanoparticles Preparation
3.6. Determination of the Yield
3.7. Extracts Encapsulation and Release
3.8. Conjugation of the Antibodies
3.9. The In Vitro Model of the Human Blood-Brain Barrier
3.10. Permeability Experiments and Cellular Accumulation
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Ladiwala, A.R.A.; Litt, J.; Kane, R.S.; Aucoin, D.S.; Smith, S.O.; Ranjan, S.; Davis, J.; van Nostrand, W.E.; Tessier, P.M. Conformational Differences between Two Amyloid beta Oligomers of Similar Size and Dissimilar Toxicity. J. Biol. Chem. 2012, 287, 24765–24773. [Google Scholar] [CrossRef] [PubMed]
- Clippingdale, A.B.; Wade, J.D.; Barrow, C.J. The amyloid-beta peptide and its role in Alzheimer’s disease. J. Pept. Sci. 2001, 7, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.H.; Checler, F. Amyloid precursor protein, presenilins, and alpha-synuclein: Molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharmacol. Rev. 2002, 54, 469–525. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.; Blennow, K. β-Amyloid (A beta) protein in cerebrospinal fluid as a biomarker for Alzheimer’s disease. Peptides 2002, 23, 1205–1214. [Google Scholar] [CrossRef]
- Citron, M.; Diehl, T.S.; Gordon, G.; Biere, A.L.; Seubert, P.; Selkoe, D.J. Evidence that the 42- and 40-amino acid forms of amyloid beta protein are generated from the β-amyloid precursor protein by different protease activities. Proc. Natl. Acad. Sci. USA 1996, 93, 13170–13175. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Lowenson, J.D.; Clarke, S.; Wolkow, C.; Wang, R.; Cotter, R.J.; Reardon, I.M.; Zurcherneely, H.A.; Heinrikson, R.L.; Ball, M.J.; et al. Structural Alterations in the Peptide Backbone of β-Amyloid Core Protein May Account for Its Deposition and Stability in Alzheimers-Disease. J. Biol. Chem. 1993, 268, 3072–3083. [Google Scholar] [PubMed]
- Evin, G.; Weidemann, A. Biogenesis and metabolism of Alzheimer's disease Abeta amyloid peptides. Peptides 2002, 23, 1285–1297. [Google Scholar] [CrossRef]
- Murphy, R.M. Peptide aggregation in neurodegenerative disease. Annu Rev. Biomed. Eng. 2002, 4, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Teplow, D.B. Amyloid beta-protein monomer folding: free-energy surfaces reveal alloform-specific differences. J. Mol. Biol. 2008, 384, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Crespo, R.; Borner, H.; Martins, P.M.; Rocha, F.A.; Coelho, M.A.N.; Pereira, M.D.; Rocha, S. Fluorinated beta-sheet breaker peptides. J. Mat. Chem B 2014, 2, 2259–2264. [Google Scholar] [CrossRef]
- Levine, H. Small molecule inhibitors of A beta assembly. Amyloid 2007, 14, 185–197. [Google Scholar] [PubMed]
- Loureiro, J.A.; Rocha, S.; Pereira, M.D. Charged surfactants induce a non-fibrillar aggregation pathway of amyloid-beta peptide. J. Pept. Sci. 2013, 19, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Lucio, M.; Lima, J.L.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- Markus, M.A.; Morris, B.J. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin. Interv. Aging 2008, 3, 331–339. [Google Scholar] [PubMed]
- Anekonda, T.S. Resveratrol—A boon for treating Alzheimer's disease? Brain Res. Rev. 2006, 52, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Inter. J. Nanomed. 2012, 7, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Lucio, M.; Martins, S.; Lima, J.L.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar]
- Neves, A.R.; Queiroz, J.F.; Costa Lima, S.A.; Figueiredo, F.; Fernandes, R.; Reis, S. Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: Relevance for oral drug delivery. J. Colloid Interface Sci. 2016, 463, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, I.; Fazakas, C.; Krizbai, I.A. In vitro models of the blood-brain barrier. Acta Neurobiol. Exp. 2011, 71, 113–128. [Google Scholar]
- Neves, A.R.; Queiroz, J.F.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Reis, S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: Two new strategies of functionalization with apolipoprotein E. Nanotechnology 2015, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood-brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, U.; Sommerfeld, P.; Ulrich, S.; Sabel, B.A. Nanoparticle Technology for Delivery of Drugs Across the Blood-Brain Barrier. J. Pharm. Sci. 1998, 87, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, G.; Salzano, G.; Caraglia, M.; Abbruzzese, A. Nanotechnologies: A Strategy to Overcome Blood-Brain Barrier. Curr. Drug. Metab. 2012, 13, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Masserini, M. Nanoparticles for Brain Drug Delivery. ISRN Biochem. 2013, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, S.B. Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases. Pharm. Res. 2013, 30, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Gomes, B.; Coelho, M.A.; Pereira, M.C.; Rocha, S. Immunoliposomes doubly targeted to transferrin receptor and to α-synuclein. Future Sci. OA 2015, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Gomes, B.; Coelho, M.A.; do Carmo Pereira, M.; Rocha, S. Targeting nanoparticles across the blood-brain barrier with monoclonal antibodies. Nanomedicine 2014, 9, 709–722. [Google Scholar] [CrossRef]
- Schnyder, A.; Krahenbuhl, S.; Torok, M.; Drewe, J.; Huwyler, J. Targeting of skeletal muscle in vitro using biotinylated immunoliposomes. Biochem. J. 2004, 377 (Pt 1), 61–67. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Gomes, B.; Fricker, G.; Cardoso, I.; Ribeiro, C.A.; Gaiteiro, C.; Coelho, M.A.; Pereira Mdo, C.; Rocha, S. Dual ligand immunoliposomes for drug delivery to the brain. Colloid. Surf. B Biointerfaces 2015, 134, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Gomes, B.; Fricker, G.; Coelho, M.A.; Rocha, S.; Pereira, M.C. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer's disease treatment. Colloid. Surf. B Biointerfaces 2016, 145, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Dodwadkar, N.S.; Sawant, R.R.; Torchilin, V.P. Development of the novel PEG-PE-based polymer for the reversible attachment of specific ligands to liposomes: Synthesis and in vitro characterization. Bioconjug. Chem. 2011, 22, 2005–2013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez-Horta, A.H.B.G.; Chavez-Montes, A. Fluorescence as a Tool to Study Lipid-Protein Interactions: The Case of α-Synuclein. Open J. Biophys. 2013, 3, 112–119. [Google Scholar] [CrossRef]
- Groenning, M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J. Chem. Biol. 2010, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, D.J.; Wranne, M.S.; Gilbert Gatty, M.; Westerlund, F.; Esbjorner, E.K. Steady-state and time-resolved Thioflavin-T fluorescence can report on morphological differences in amyloid fibrils formed by Abeta(1-40) and Abeta(1-42). Biochem. Biophys. Res. Commun. 2015, 458, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Loureiro, J.A.; Brezesinski, G.; Pereira, M.D. Peptide-surfactant interactions: Consequences for the amyloid-beta structure. Biochem. Biopys. Res. Commun. 2012, 420, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hung, V.W.S.; Cheng, X.R.; Li, N.; Veloso, A.J.; Kerman, K. Electrochemical Detection of Amyloid-Beta Aggregation in the Presence of Resveratrol. J. Electrochem. Soc. 2013, 160, G3097–G3101. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol Promotes Clearance of Alzheimer's Disease Amyloid-beta Peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Martins, S.; Segundo, M.A.; Reis, S. Nanoscale Delivery of Resveratrol towards Enhancement of Supplements and Nutraceuticals. Nutrients 2016, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Markoutsa, E.; Pampalakis, G.; Niarakis, A.; Romero, I.A.; Weksler, B.; Couraud, P.O.; Antimisiaris, S.G. Uptake and permeability studies of BBB-targeting immunoliposomes using the hCMEC/D3 cell line. Eur. J. Pharm. Biopharm. 2011, 77, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Tho, I.; Souto, E.; Ferreira, D.; Brandl, M. Multivariate design for the evaluation of lipid and surfactant composition effect for optimisation of lipid nanoparticles. Eur. J. Pharm. Sci. 2012, 45, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 2). Trop. J. Pharm. Res. 2013, 12, 8. [Google Scholar]
- Mason, J.M.; Kokkoni, N.; Stott, K.; Doig, A.J. Design strategies for anti-amyloid agents. Curr. Opin. Struct. Biol. 2003, 13, 526–532. [Google Scholar] [CrossRef]
- Henry, N.; Parce, J.W.; McConnell, H.M. Visualization of specific antibody and C1q binding to hapten-sensitized lipid vesicles. Proc. Natl. Acad. Sci. USA 1978, 75, 3933–3937. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Aday, S.; Sevin, E.; Almeida, C.; Culot, M.; Dehouck, L.; Coisne, C.; Engelhardt, B.; Dehouck, M.P.; Ferreira, L. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS ONE 2014, 9, e99733. [Google Scholar] [CrossRef] [PubMed]
- LeVine, H., III. Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: San Diego, CA, USA, 1996; p. 785. [Google Scholar]
- Pardridge, W.M.; Kang, Y.S.; Buciak, J.L.; Yang, J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm. Res. 1995, 12, 807–886. [Google Scholar] [CrossRef] [PubMed]
- Oller-Salvia, B.; Sanchez-Navarro, M.; Ciudad, S.; Guiu, M.; Arranz-Gibert, P.; Garcia, C.; Gomis, R.R.; Cecchelli, R.; Garcia, J.; Giralt, E.; et al. MiniAp-4: A Venom-Inspired Peptidomimetic for Brain Delivery. Angew. Chem. 2016, 55, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, D.C.; Tellechea, A.; Moura, L.; Fidalgo-Carvalho, I.; Duarte, J.; Carvalho, E.; Ferreira, L. Improved survival, vascular differentiation and wound healing potential of stem cells co-cultured with endothelial cells. PLoS ONE 2011, 6, e16114. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Dehouck, B.; Descamps, L.; Fenart, L.; Buee-Scherrer, V.V.; Duhem, C.; Lundquist, S.; Rentfel, M.; Torpier, G.; Dehouck, M.P. In vitro model for evaluating drug transport across the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 165–178. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the extracts of the grape seed and grape skin are available from the authors JAL and MCP.






| Encapsulated Extract | Quantity of Extract (mg) | |||
| 2 | 5 | 10 | 15 | |
| Nanoparticle Diameter (nm) | ||||
| Grape skin | 187 ± 3 | 184 ± 8 | 182 ± 6 | 188 ± 18 |
| Grape seed | 168 ± 10 | 174 ± 12 | 188 ± 9 | 189 ± 2 |
| Encapsulated Extract | Quantity of Extract (mg) | |||
| 2 | 5 | 10 | 15 | |
| Entrapment Efficiency (%) | ||||
| Grape skin | 100 ± 20 | 100 ± 12 | 92 ± 7 | 75 ± 7 |
| Grape seed | 97 ± 2 | 86 ± 27 | 95 ± 2 | 97 ± 2 |
| SLN | Size (nm) | Zeta Potential (mV) | Entrapment Efficiency (%) | |||
|---|---|---|---|---|---|---|
| 0 day | 2 months | 0 day | 2 months | 0 day | 2 months | |
| Unloaded | 142 ± 10 | 172 ± 3 | −0.08 | −0.21 | - | - |
| Grape skin | 182 ± 6 | 166 ± 10 | −0.07 | −0.02 | 92 ± 7 | 88 ± 10 |
| Grape seed | 188 ± 9 | 197 ± 20 | 0.34 | −0.04 | 95 ± 2 | 97 ± 3 |
| SLN | Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| Without mab | 233 ± 10 | 0.13 ± 0.03 | −5.4 ± 0.5 |
| With LB 509 mab | 249 ± 1 | 0.14 ± 0.05 | −5.0 ± 0.1 |
| With OX26 mab | 254 ± 17 | 0.23 ± 0.05 | −4.0 ± 0.1 |
| Name | Structure |
|---|---|
| Cetylpalmitate | 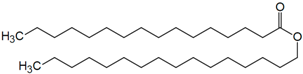 |
| Polysorbate 80 | 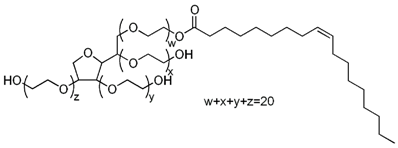 |
| DSPE-PEG(2000) Maleimide: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(maleimide(polyethyleneglycol)-2000) | 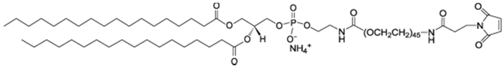 |
| Liss Rhod PE: 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) | 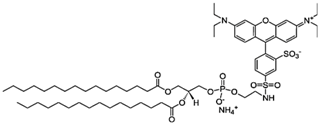 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.N.; et al. Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 277. https://doi.org/10.3390/molecules22020277
Loureiro JA, Andrade S, Duarte A, Neves AR, Queiroz JF, Nunes C, Sevin E, Fenart L, Gosselet F, Coelho MAN, et al. Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules. 2017; 22(2):277. https://doi.org/10.3390/molecules22020277
Chicago/Turabian StyleLoureiro, Joana A., Stephanie Andrade, Ana Duarte, Ana Rute Neves, Joana Fontes Queiroz, Cláudia Nunes, Emmanuel Sevin, Laurence Fenart, Fabien Gosselet, Manuel A. N. Coelho, and et al. 2017. "Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease" Molecules 22, no. 2: 277. https://doi.org/10.3390/molecules22020277
APA StyleLoureiro, J. A., Andrade, S., Duarte, A., Neves, A. R., Queiroz, J. F., Nunes, C., Sevin, E., Fenart, L., Gosselet, F., Coelho, M. A. N., & Pereira, M. C. (2017). Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules, 22(2), 277. https://doi.org/10.3390/molecules22020277