Zerumbone Alleviates Neuropathic Pain through the Involvement of l-Arginine-Nitric Oxide-cGMP-K+ ATP Channel Pathways in Chronic Constriction Injury in Mice Model
Abstract
:1. Introduction
2. Results
2.1. l-Arginine-Nitric Oxide Pathway
2.2. Cyclic Guanosine Monophosphate (cGMP) Pathway
2.3. Potassium (K+) Channel Pathway
2.4. Rota Rod Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Surgical Procedure
4.3. Preparation of Zerumbone for Experiments
4.4. Preparation of Drugs and Chemicals
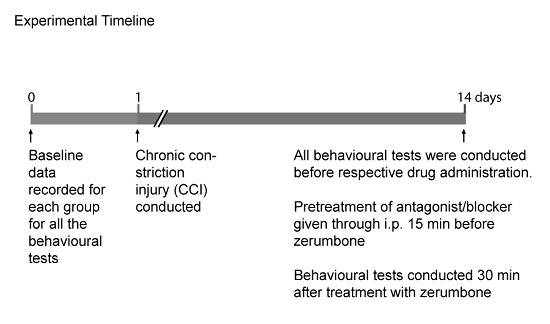
4.5. Groups and Timeline
4.6. Allodynia Effect
4.7. Hyperalgesia Effect
4.8. Rota Rod Analysis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tsuda, M.; Inoue, K.; Salter, M.W. Neuropathic pain and spinal microglia: A big problem from molecules in “small” glia. Trends Neurosci. 2005, 28, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Kalso, E.A.; Loeser, J.D.; Miaskowski, C.; Nurmikko, T.J.; et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Gottrup, H.; Sindrup, S.H.; Bach, F.W. The clinical picture of neuropathic pain. Eur. J. Pharmacol. 2001, 429, 1–11. [Google Scholar] [CrossRef]
- Dowdall, T.; Robinson, I.; Meert, T.F. Comparison of five different rat models of peripheral nerve injury. Pharmacol. Biochem. Behav. 2005, 80, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rosberg, K.; Kvarnstrom, A.; Kinnman, E.; Gordh, T.; Nordfors, L.O.; Kristofferson, A. Peripheral neuropathic pain—A multidimensional burden for patients. Eur. J. Pain 2001, 5, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Yob, N.J.; Jofrry, S.M.; Affandi, M.M.; Teh, L.K.; Salleh, M.Z.; Zakaria, Z.A. Zingiber zerumbet (L.) Smith: A Review of Its Ethnomedicinal, Chemical, and Pharmacological Uses. Evid. Based Complement. Altern. Med. 2011, 2011, 543216. [Google Scholar] [CrossRef] [PubMed]
- Perimal, E.K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Ming, O.H.; Khalid, S.; Tatt, L.M.; Kamaldin, M.N.; Zakaria, Z.A.; Israf, D.A.; et al. Zerumbone-induced antinociception: Involvement of the l-arginine-nitric oxide-cGMP -PKC-K+ ATP channel pathways. Basic Clin. Pharmacol. Toxicol. 2011, 108, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.R.; Tengku Mohamad, T.A.; Shaik Mossadeq, W.M.; Moin, S.; Yusof, M.; Mokhtar, A.F.; Zakaria, Z.A.; Israf, D.A.; Lajis, N. Antinociceptive activity of the essential oil of Zingiber zerumbet. Planta Med. 2010, 76, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Takahashi, D.; Kinoshita, T.; Koshimizu, K.; Kim, H.W.; Yoshihiro, A.; Nakamura, Y.; Jiwajinda, S.; Terao, J.; Ohigashi, H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: The alpha,beta-unsaturated carbonyl group is a prerequisite. Carcinogenesis 2002, 23, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.C.; Chien, T.Y.; Chen, L.G.; Wang, C.C. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005, 71, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Zulazmi, N.A.; Gopalsamy, B.; Omar Farouk, A.A.; Sulaiman, M.R.; Bharatham, B.H.; Perimal, E.K. Antiallodynic and antihyperalgesic effects of zerumbone on a mouse model of chronic constriction injury-induced neuropathic pain. Fitoterapia 2015, 105, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.; Omar Farouk, A.A.; Mohamad, A.S.; Sulaiman, M.R.; Perimal, E.K. Zerumbone alleviates chronic constriction injury-induced allodynia and hyperalgesia through serotonin 5-HT receptors. Biomed. Pharmacother. 2016, 83, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S. Nitric oxide in the vasculature: Physiology and pathophysiology. Ann. N. Y. Acad. Sci. 1997, 811, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.T.; Gebhart, G.F. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain 1993, 52, 127–136. [Google Scholar] [CrossRef]
- Garthwaite, J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991, 14, 60–67. [Google Scholar] [CrossRef]
- Haley, J.E.; Dickenson, A.H.; Schachter, M. Electrophysiological evidence for a role of nitric oxide in prolonged chemical nociception in the rat. Neuropharmacology 1992, 31, 251–258. [Google Scholar] [CrossRef]
- Bredt, D.S.; Snyder, S.H. Nitric oxide, a novel neuronal messenger. Neuron 1992, 8, 3–11. [Google Scholar] [CrossRef]
- Garry, M.G.; Walton, L.P.; Davis, M.A. Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from the spinal cord is mediated by nitric oxide but not by cyclic GMP. Brain Res. 2000, 861, 208–219. [Google Scholar] [CrossRef]
- Kiss, J.P.; Vizi, E.S. Nitric oxide: A novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001, 24, 211–215. [Google Scholar] [CrossRef]
- Fiallos-Estrada, C.E.; Kummer, W.; Mayer, B.; Bravo, R.; Zimmermann, M.; Herdegen, T. Long-lasting increase of nitric oxide synthase immunoreactivity, NADPH-diaphorase reaction and c-JUN co-expression in rat dorsal root ganglion neurons following sciatic nerve transection. Neurosci. Lett. 1993, 150, 169–173. [Google Scholar] [CrossRef]
- Wu, W. Expression of nitric-oxide synthase (NOS) in injured CNS neurons as shown by NADPH diaphorase histochemistry. Exp. Neurol. 1993, 120, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Hoke, A.; Zochodne, D.W. Local expression of inducible nitric oxide synthase in an animal model of neuropathic pain. Neurosci. Lett. 1999, 260, 207–209. [Google Scholar] [CrossRef]
- Martucci, C.; Trovato, A.E.; Costa, B.; Borsani, E.; Franchi, S.; Magnaghi, V.; Panerai, A.E.; Rodella, L.F.; Valsecchi, A.E.; Sacerdote, P.; et al. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain 2008, 137, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; Negrete, R.; Leanez, S.; Martin-Campos, J.M.; Pol, O. The spinal cord expression of neuronal and inducible nitric oxide synthases and their contribution in the maintenance of neuropathic pain in mice. PLoS ONE 2010, 5, e14321. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.D.; Ferreira, S.H. The molecular mechanism of central analgesia induced by morphine or carbachol and the l-arginine-nitric oxide-cGMP pathway. Eur. J. Pharmacol. 1992, 221, 171–174. [Google Scholar] [CrossRef]
- Bolotina, V.M.; Najibi, S.; Palacino, J.J.; Pagano, P.J.; Cohen, R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 1994, 368, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.A.; Pitari, G.M.; Kazerounian, S.; Ruiz-Stewart, I.; Park, J.; Schulz, S.; Chepenik, K.P.; Waldman, S.A. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 2000, 52, 375–414. [Google Scholar] [PubMed]
- Han, J.; Kim, N.; Joo, H.; Kim, E.; Earm, Y.E. ATP-sensitive K(+) channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1545–H1554. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Zochodne, D.W. NO pain: Potential roles of nitric oxide in neuropathic pain. Pain Pract. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ocana, M.; Cendan, C.M.; Cobos, E.J.; Entrena, J.M.; Baeyens, J.M. Potassium channels and pain: Present realities and future opportunities. Eur. J. Pharmacol. 2004, 500, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Patil, C.S.; Singh, A.; Kulkarni, S.K. Sildenafil, a phosphodiesterase-5 inhibitor, enhances the antinociceptive effect of morphine. Pharmacology 2003, 67, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Yamazumi, I.; Okuda, T.; Koga, Y. Involvement of potassium channels in spinal antinociceptions induced by fentanyl, clonidine and bethanechol in rats. Jpn. J. Pharmacol. 2001, 87, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kitto, K.F.; Haley, J.E.; Wilcox, G.L. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci. Lett. 1992, 148, 1–5. [Google Scholar] [CrossRef]
- Callsen-Cencic, P.; Hoheisel, U.; Kaske, A.; Mense, S.; Tenschert, S. The controversy about spinal neuronal nitric oxide synthase: Under which conditions is it up- or downregulated? Cell Tissue Res. 1999, 295, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Mukai, Y.; Shimokawa, H.; Takeshita, A. Atorvastatin causes depressor and sympatho-inhibitory effects with upregulation of nitric oxide synthases in stroke-prone spontaneously hypertensive rats. J. Hypertens. 2003, 21, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.C.; Guan, Y.; Skinner, J.; Raja, S.N.; Johns, R.A.; Tao, Y.X. Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund’s adjuvant-induced persistent pain. Pain 2005, 119, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Svensson, C.I.; Fitzsimmons, B.; Webb, M.; Yaksh, T.L.; Hua, X.Y. Inhibition of spinal constitutive NOS-2 by 1400W attenuates tissue injury and inflammation-induced hyperalgesia and spinal p38 activation. Eur. J. Neurosci. 2007, 25, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.K.; Tandan, S.K.; Kumar, D.; Dudhgaonkar, S.P. Nitric oxide and its modulators in chronic constriction injury-induced neuropathic pain in rats. Eur. J. Pharmacol. 2006, 530, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Zoga, V.; Kimura, M.; Liang, M.Y.; Wu, H.E.; Gemes, G.; McCallum, J.B.; Kwok, W.M.; Hogan, Q.H.; Sarantopoulos, C.D. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: Action by direct S-nitrosylation. Mol. Pain 2009, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Boger, R.H.; Bode-Boger, S.M. The clinical pharmacology of l-arginine. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Kamaldin, M.N.; Akhtar, M.N.; Mohamad, A.S.; Lajis, N.; Perimal, E.K.; Akira, A.; Ming-Tatt, L.; Israf, D.A.; Sulaiman, M.R. Peripheral antinociception of a chalcone, flavokawin B and possible involvement of the nitric oxide/cyclic guanosine monophosphate/potassium channels pathway. Molecules 2013, 18, 4209–4220. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.R.; Williams, T.J.; Brain, S.D. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur. J. Pharmacol. 1990, 191, 481–484. [Google Scholar] [CrossRef]
- Ialenti, A.; Ianaro, A.; Moncada, S.; Di Rosa, M. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharmacol. 1992, 211, 177–182. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Leopold, E.; Schmidt, K.; Brunner, F.; Mayer, B. Inhibition of nitric oxide synthesis by NG-nitro-l-arginine methyl ester (l-NAME): Requirement for bioactivation to the free acid, NG-nitro-l-arginine. Br. J. Pharmacol. 1996, 118, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi-Fard, M.A.; Fung, H.L. Pharmacokinetics, plasma protein binding and urinary excretion of N omega-nitro-l-arginine in rats. Br. J. Pharmacol. 1994, 111, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Santos, A.R. S.; Calixto, J.B. The role of systemic, spinal and supraspinal l-arginine–nitric oxide–cGMP pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology 1999, 38, 835–842. [Google Scholar] [CrossRef]
- Sousa, A.M.; Prado, W.A. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001, 897, 9–19. [Google Scholar] [CrossRef]
- Pottabathini, R.; Kumar, A.; Bhatnagar, A.; Garg, S. Possible Involvement of Nitric Oxide Modulatory Mechanism in the Protective Effect of Retigabine Against Spinal Nerve Ligation-Induced Neuropathic Pain. Cell. Mol. Neurobiol. 2015, 35, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Makuch, W.; Mika, J.; Rojewska, E.; Zychowska, M.; Przewlocka, B. Effects of selective and non-selective inhibitors of nitric oxide synthase on morphine- and endomorphin-1-induced analgesia in acute and neuropathic pain in rats. Neuropharmacology 2013, 75, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.C.; Lv, Y.W.; Ji, Y.; Yan, X.J.; Xue, J.P. Involvement of the nitric oxide-cyclic GMP-protein kinase G-K+ channel pathway in the antihyperalgesic effects of bovine lactoferrin in a model of neuropathic pain. Brain Res. 2008, 1209, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fesenko, E.E.; Kolesnikov, S.S.; Lyubarsky, A.L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 1985, 313, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Raab-Graham, K.; Jan, Y.N.; Jan, L.Y. NO stimulation of ATP-sensitive potassium channels: Involvement of Ras/mitogen-activated protein kinase pathway and contribution to neuroprotection. Proc. Natl. Acad. Sci. USA 2004, 101, 7799–7804. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Hare, J.M. The role of nitric oxide in the physiological regulation of Ca2+ cycling. Curr. Opin. Drug Discov. Dev. 2003, 6, 658–666. [Google Scholar]
- Cahusac, P.M.; Evans, R.H.; Hill, R.G.; Rodriquez, R.E.; Smith, D.A. The behavioural effects of an N-methylaspartate receptor antagonist following application to the lumbar spinal cord of conscious rats. Neuropharmacology 1984, 23, 719–724. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Lorenzetti, B.B.; Ferreira, S.H. Activation of the arginine-nitric oxide pathway in primary sensory neurons contributes to dipyrone-induced spinal and peripheral analgesia. Inflamm. Res. 1996, 45, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Deciga-Campos, M.; Lopez-Munoz, F.J. Participation of the l-arginine-nitric oxide-cyclic GMP-ATP-sensitive K+ channel cascade in the antinociceptive effect of rofecoxib. Eur. J. Pharmacol. 2004, 484, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, V.P.; Zambelli, V.O.; Picolo, G.; Chacur, M.; Sampaio, S.C.; Brigatte, P.; Konno, K.; Cury, Y. The peripheral l-arginine-nitric oxide-cyclic GMP pathway and ATP-sensitive K(+) channels are involved in the antinociceptive effect of crotalphine on neuropathic pain in rats. Behav. Pharmacol. 2012, 23, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.H.; Akhtar, M.N.; Mohamad, A.S.; Perimal, E.K.; Akira, A.; Israf, D.A.; Lajis, N.; Sulaiman, M.R. Antinociceptive effect of the essential oil of Zingiber zerumbet in mice: Possible mechanisms. J. Ethnopharmacol. 2011, 137, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Min, H.Y.; Kim, M.S.; Han, A.R.; Windono, T.; Jeohn, G.H.; Kang, S.S.; Lee, S.K.; Seo, E.K. Humulene derivatives from Zingiber zerumbet with the inhibitory effects on lipopolysaccharide-induced nitric oxide production. Chem. Pharm. Bull. 2005, 53, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Yaster, M.; Raja, S.N.; Tao, Y.X. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol. Pain 2007, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Kubes, P.; Zochodne, D.W. Delayed peripheral nerve degeneration, regeneration, and pain in mice lacking inducible nitric oxide synthase. J. Neuropathol. Exp. Neurol. 2001, 60, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Zochodne, D.W. Local nitric oxide synthase activity in a model of neuropathic pain. Eur. J. Neurosci. 1998, 10, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- De Alba, J.; Clayton, N.M.; Collins, S.D.; Colthup, P.; Chessell, I.; Knowles, R.G. GW274150, a novel and highly selective inhibitor of the inducible isoform of nitric oxide synthase (iNOS), shows analgesic effects in rat models of inflammatory and neuropathic pain. Pain 2006, 120, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; Ren, K.; Besse, D.; Ruda, M.A.; Dubner, R. Application of nitric oxide synthase inhibitor, N omega-nitro-l-arginine methyl ester, on injured nerve attenuates neuropathy-induced thermal hyperalgesia in rats. Neurosci. Lett. 1996, 210, 124–126. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lin, D.P.; Wu, C.Y.; Teng, M.C.; Sun, C.Y.; Tsai, Y.T.; Su, K.C.; Wang, S.R.; Chang, H.H. Dietary zerumbone prevents mouse cornea from UVB-induced photokeratitis through inhibition of NF-kappaB, iNOS, and TNF-alpha expression and reduction of MDA accumulation. Mol. Vis. 2011, 17, 854–863. [Google Scholar] [PubMed]
- Sachs, D.; Cunha, F.Q.; Ferreira, S.H. Peripheral analgesic blockade of hypernociception: Activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 3680–3685. [Google Scholar] [CrossRef] [PubMed]
- Vale, M.L.; Rolim, D.E.; Cavalcante, I.F.; Ribeiro, R.A.; Souza, M.H. Role of NO/cGMP/KATP pathway in antinociceptive effect of sildenafil in zymosan writhing response in mice. Inflamm. Res. 2007, 56, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Ming-Tatt, L.; Khalivulla, S.I.; Akhtar, M.N.; Lajis, N.; Perimal, E.K.; Akira, A.; Ali, D.I.; Sulaiman, M.R. Anti-hyperalgesic effect of a benzilidine-cyclohexanone analogue on a mouse model of chronic constriction injury-induced neuropathic pain: Participation of the κ-opioid receptor and K ATP. Pharmacol. Biochem. Behav. 2013, 114, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Martinov, T.; Mack, M.; Sykes, A.; Chatterjea, D. Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von Frey apparatus. J. Vis. Exp. 2013, e51212. [Google Scholar] [CrossRef] [PubMed]
- Santos-Nogueira, E.; Redondo Castro, E.; Mancuso, R.; Navarro, X. Randall-Selitto test: A new approach for the detection of neuropathic pain after spinal cord injury. J. Neurotrauma 2012, 29, 898–904. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |








| Experimental Groups (n = 6) | Dose | Experimental Conditions |
|---|---|---|
| Sham | - | Without ligature to the nerve and no treatment |
| Vehicle (mL/kg, i.p.) | 10 | Subjected to CCI and treated with vehicle |
| Zerumbone (mg/kg, i.p.) | 10 | Subjected to CCI and treated with zerumbone |
| l-arginine (mg/kg, i.p.) | 10 | Subjected to CCI and pre-treated with different antagonist and agonist |
| l-NOARG (mg/kg, i.p.) | 10 | |
| l-arginine + l-NOARG | 10 + 10 | |
| l-arginine + Zerumbone | 10 + 10 | |
| l-NOARG + Zerumbone | 10 + 10 | |
| ODQ (mg/kg, i.p.) | 2 | Subjected to CCI and pre-treated with ODQ |
| ODQ + Zerumbone | 2 + 10 | |
| Glibenclamide (mg/kg, i.p.) | 10 | Subjected to CCI and pre-treated with Glibenclamide |
| Glibenclamide + Zerumbone | 10 + 10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulazmi, N.A.; Gopalsamy, B.; Min, J.C.S.; Farouk, A.A.O.; Sulaiman, M.R.; Bharatham, B.H.; Perimal, E.K. Zerumbone Alleviates Neuropathic Pain through the Involvement of l-Arginine-Nitric Oxide-cGMP-K+ ATP Channel Pathways in Chronic Constriction Injury in Mice Model. Molecules 2017, 22, 555. https://doi.org/10.3390/molecules22040555
Zulazmi NA, Gopalsamy B, Min JCS, Farouk AAO, Sulaiman MR, Bharatham BH, Perimal EK. Zerumbone Alleviates Neuropathic Pain through the Involvement of l-Arginine-Nitric Oxide-cGMP-K+ ATP Channel Pathways in Chronic Constriction Injury in Mice Model. Molecules. 2017; 22(4):555. https://doi.org/10.3390/molecules22040555
Chicago/Turabian StyleZulazmi, Nurul Atiqah, Banulata Gopalsamy, Jasmine Chia Siew Min, Ahmad Akira Omar Farouk, Mohd Roslan Sulaiman, B. Hemabarathy Bharatham, and Enoch Kumar Perimal. 2017. "Zerumbone Alleviates Neuropathic Pain through the Involvement of l-Arginine-Nitric Oxide-cGMP-K+ ATP Channel Pathways in Chronic Constriction Injury in Mice Model" Molecules 22, no. 4: 555. https://doi.org/10.3390/molecules22040555
APA StyleZulazmi, N. A., Gopalsamy, B., Min, J. C. S., Farouk, A. A. O., Sulaiman, M. R., Bharatham, B. H., & Perimal, E. K. (2017). Zerumbone Alleviates Neuropathic Pain through the Involvement of l-Arginine-Nitric Oxide-cGMP-K+ ATP Channel Pathways in Chronic Constriction Injury in Mice Model. Molecules, 22(4), 555. https://doi.org/10.3390/molecules22040555