Discovery of an Octahedral Silicon Complex as a Potent Antifungal Agent
Abstract
:1. Introduction
2. Results and Discussion
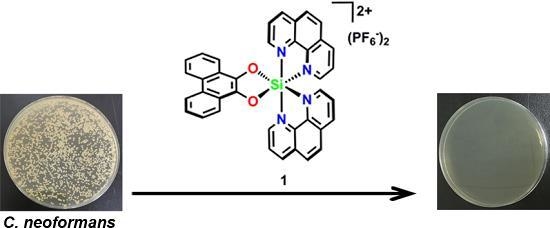
2.1. Antimicrobial Activity
2.2. Determination of MIC Values
2.3. Determination of MFC Value of Complex 1
2.4. A Comparative Time-Course Assay of Cell Viability
2.5. Antifungal Mechanism
3. Materials and Methods
3.1. Syntheses
3.2. Disk Diffusion Susceptibility Test
3.3. Determination of MIC and MFC Values
3.4. Anti-Fungal Activity of Complex 1 on C. neoformans in the Presence of ssDNA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- May, R.C.; Stone, N.R.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with hiv/aids. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Bicanic, T.; Harrison, T.; Niepieklo, A.; Dyakopu, N.; Meintjes, G. Symptomatic relapse of hiv-associated cryptococcal meningitis after initial fluconazole monotherapy: The role of fluconazole resistance and immune reconstitution. Clin. Infect. Dis. 2006, 43, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Sionov, E.; Chang, Y.C.; Kwon-Chung, K.J. Azole heteroresistance in cryptococcus neoformans: Emergence of resistant clones with chromosomal disomy in the mouse brain during fluconazole treatment. Antimicrob. Agents Chemother. 2013, 57, 5127–5130. [Google Scholar] [CrossRef] [PubMed]
- Yamazumi, T.; Pfaller, M.A.; Messer, S.A.; Houston, A.K.; Boyken, L.; Hollis, R.J.; Furuta, I.; Jones, R.N. Characterization of heteroresistance to fluconazole among clinical isolates of cryptococcus neoformans. J. Clin. Microbiol. 2003, 41, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Sanguinetti, M.; Sanglard, D.; La Sorda, M.; Boccia, S.; Romano, L.; Morace, G.; Fadda, G. Identification and characterization of a cryptococcus neoformans atp binding cassette (abc) transporter-encoding gene, cnafr1, involved in the resistance to fluconazole. Mol. Microbiol. 2003, 47, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, R. Novel agents for antimicrobial solutions. Eye Contact Lens Sci. Clin. Pract. 2016, 42, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Pothiraj, K.; Baskaran, T. DNA-binding, oxidative DNA cleavage, and coordination mode of later 3d transition metal complexes of a schiff base derived from isatin as antimicrobial agents. J. Coord. Chem. 2011, 64, 3900–3917. [Google Scholar] [CrossRef]
- Xiang, Y.; Fu, C.; Breiding, T.; Sasmal, P.K.; Liu, H.; Shen, Q.; Harms, K.; Zhang, L.; Meggers, E. Hydrolytically stable octahedral silicon complexes as bioactive scaffolds: Application to the design of DNA intercalators. Chem. Commun. 2012, 48, 7131–7133. [Google Scholar] [CrossRef] [PubMed]
- Henker, J.; Glockner, S.; Meggers, E. Dinuclear ruthenium-silicon complexes as DNA and g4 DNA binding agents. J. Biol. Inorg. Chem. 2014, 19, S367. [Google Scholar]
- Fu, C.; Harms, K.; Zhang, L.L.; Meggers, E. DNA mismatch recognition by a hexacoordinate silicon sandwich-ruthenium hybrid complex. Organometallics 2014, 33, 3219–3222. [Google Scholar] [CrossRef]
- Kummer, D.; Gaisser, K.E.; Seshadri, T. Contributions to chemistry of halosilane adducts.10. Bis(2,2′-bipyridine) complexes of silicon, direct synthesis, structure, and properties. Chem. Ber. Recl. 1977, 110, 1950–1962. [Google Scholar] [CrossRef]
- Peloquin, D.M.; Schmedake, T.A. Recent advances in hexacoordinate silicon with pyridine-containing ligands: Chemistry and emerging applications. Coord. Chem. Rev. 2016, 323, 107–119. [Google Scholar] [CrossRef]
- Erkkila, K.E.; Odom, D.T.; Barton, J.K. Recognition and reaction of metallointercalators with DNA. Chem. Rev. 1999, 99, 2777–2795. [Google Scholar] [CrossRef] [PubMed]
- Hannon, M.J. Supramolecular DNA recognition. Chem. Soc. Rev. 2007, 36, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Keene, F.R.; Smith, J.A.; Collins, J.G. Metal complexes as structure-selective binding agents for nucleic acids. Coord. Chem. Rev. 2009, 253, 2021–2035. [Google Scholar] [CrossRef]
- Liu, H.K.; Sadler, P.J. Metal complexes as DNA intercalators. Acc. Chem. Res. 2011, 44, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Lu, L.; Liu, L.J.; Chao, W.C.; Zhong, H.J.; Wang, M.; Chen, X.P.; Lu, J.J.; Li, R.N.; Ma, D.L.; Leung, C.H. Identification of an iridium(iii) complex with anti-bacterial and anti-cancer activity. Sci. Rep. 2015, 5, 14544. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Wu, C.; Wang, L.; Sachs, M.S.; Lin, X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 2012, 56, 3758–3766. [Google Scholar] [CrossRef] [PubMed]
- Gorle, A.K.; Feterl, M.; Warner, J.M.; Wallace, L.; Keene, F.R.; Collins, J.G. Tri- and tetra-nuclear polypyridyl ruthenium(ii) complexes as antimicrobial agents. Dalton Trans. 2014, 43, 16713–16725. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 539, 314. [Google Scholar] [CrossRef] [PubMed]
- Calderazzo, F.; Forte, C.; Marchetti, F.; Pampaloni, G.; Pieretti, L. Reaction of phenanthrene-9,10-dione with phenanthrene-9,10-diol: Synthesis and characterization of the first ortho-quinhydrone derivative. Helv. Chim. Acta 2004, 87, 781–789. [Google Scholar] [CrossRef]
- Ye, R.R.; Ke, Z.F.; Tan, C.P.; He, L.; Ji, L.N.; Mao, Z.W. Histone-deacetylase-targeted fluorescent ruthenium(ii) polypyridyl complexes as potent anticancer agents. Chem. A Eur. J. 2013, 19, 10160–10169. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, G.; Dongare, P.; Dawe, L.N.; Thompson, D.W.; Zhao, Y.M.; Bodwell, G.J. 1,8-Pyrenylene-ethynylene macrocycles. Org. Lett. 2011, 13, 2240–2243. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the complexes 1–3 are available from the authors. |





| Drugs | C. neoformans |
|---|---|
| 1 | 4.5 |
| 2 | 34.3 |
| 3 | 74.4 |
| fluconazole | 6.5 |
| amphotericin B | 0.3 |
| 5-flucytosine | 3.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Fu, B.; Peng, X.; Liao, G. Discovery of an Octahedral Silicon Complex as a Potent Antifungal Agent. Molecules 2017, 22, 637. https://doi.org/10.3390/molecules22040637
Fu C, Fu B, Peng X, Liao G. Discovery of an Octahedral Silicon Complex as a Potent Antifungal Agent. Molecules. 2017; 22(4):637. https://doi.org/10.3390/molecules22040637
Chicago/Turabian StyleFu, Chen, Bin Fu, Xixi Peng, and Guojian Liao. 2017. "Discovery of an Octahedral Silicon Complex as a Potent Antifungal Agent" Molecules 22, no. 4: 637. https://doi.org/10.3390/molecules22040637
APA StyleFu, C., Fu, B., Peng, X., & Liao, G. (2017). Discovery of an Octahedral Silicon Complex as a Potent Antifungal Agent. Molecules, 22(4), 637. https://doi.org/10.3390/molecules22040637