The Transcriptome of Type I Murine Astrocytes under Interferon-Gamma Exposure and Remyelination Stimulus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Astrocyte Transcriptional Markers
2.2. Transcriptome Profiling of Astrocytes upon IFNγ and Benztropine Exposure
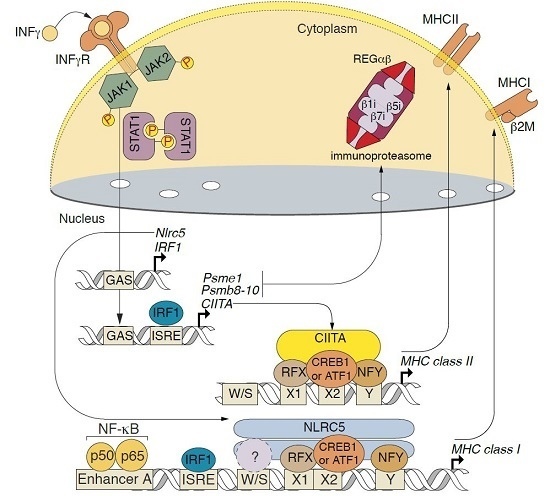
2.3. Changes in Transcription of Genes Related to Antigen Proteolysis and Presentation in Astrocytes in Response to IFNγ
3. Materials and Methods
3.1. Astrocytes Culture
3.2. Microarray Analyses
3.3. qPCR Validation of Gene Expression Data
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple sclerosis. N. Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Farez, M.F. The role of astrocytes in multiple sclerosis progression. Front. Neurol. 2015, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Arellano, G.; Ottum, P.A.; Reyes, L.I.; Burgos, P.I.; Naves, R. Stage-specific role of interferon-gamma in experimental autoimmune encephalomyelitis and multiple sclerosis. Front. Immunol. 2015, 6, 492. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.A.; Tardif, V.; Lyssiotis, C.A.; Green, C.C.; Kerman, B.; Kim, H.J.; Padmanabhan, K.; Swoboda, J.G.; Ahmad, I.; Kondo, T.; et al. A regenerative approach to the treatment of multiple sclerosis. Nature 2013, 502, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; You, Y.; Wu, J.Q. Building an RNA sequencing transcriptome of the central nervous system. Neuroscientist 2016, 22, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Schmitt, S.; Bergner, C.G.; Tyanova, S.; Kannaiyan, N.; Manrique-Hoyos, N.; Kongi, K.; Cantuti, L.; Hanisch, U.K.; Philips, M.A.; et al. Cell type- and brain region-resolved mouse brain proteome. Nat. Neurosci. 2015, 18, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Patzig, J.; Jahn, O.; Tenzer, S.; Wichert, S.P.; de Monasterio-Schrader, P.; Rosfa, S.; Kuharev, J.; Yan, K.; Bormuth, I.; Bremer, J.; et al. Quantitative and integrative proteome analysis of peripheral nerve myelin identifies novel myelin proteins and candidate neuropathy loci. J. Neurosci. 2011, 31, 16369–16386. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Munoz-Manchado, A.B.; Codeluppi, S.; Lonnerberg, P.; La Manno, G.; Jureus, A.; Marques, S.; Munguba, H.; He, L.; Betsholtz, C.; et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 2015, 347, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Gokce, O.; Stanley, G.M.; Treutlein, B.; Neff, N.F.; Camp, J.G.; Malenka, R.C.; Rothwell, P.E.; Fuccillo, M.V.; Sudhof, T.C.; Quake, S.R. Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-seq. Cell Rep. 2016, 16, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Holtman, I.R.; Noback, M.; Bijlsma, M.; Duong, K.N.; van der Geest, M.A.; Ketelaars, P.T.; Brouwer, N.; Vainchtein, I.D.; Eggen, B.J.; Boddeke, H.W. Glia open access database (goad): A comprehensive gene expression encyclopedia of glia cells in health and disease. Glia 2015, 63, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Thakurela, S.; Garding, A.; Jung, R.B.; Muller, C.; Goebbels, S.; White, R.; Werner, H.B.; Tiwari, V.K. The transcriptome of mouse central nervous system myelin. Sci. Rep. 2016, 6, 25828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O'Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Aaker, J.D.; Elbaz, B.; Wu, Y.; Looney, T.J.; Zhang, L.; Lahn, B.T.; Popko, B. Transcriptional fingerprint of hypomyelination in zfp191null and shiverer (mbpshi) mice. ASN Neuro 2016, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ettle, B.; Kerman, B.E.; Valera, E.; Gillmann, C.; Schlachetzki, J.C.; Reiprich, S.; Buttner, C.; Ekici, A.B.; Reis, A.; Wegner, M.; et al. Alpha-synuclein-induced myelination deficit defines a novel interventional target for multiple system atrophy. Acta Neuropathol. 2016, 132, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Kudriaeva, A.A.; Khaustova, N.A.; Maltseva, D.V.; Kuzina, E.S.; Glagoleva, I.S.; Surina, E.A.; Knorre, V.D.; Belogurov, A.A., Jr.; Tonevitsky, A.G.; Gabibov, A.G. Mrna expression profile of mouse oligodendrocytes in inflammatory conditions. Dokl. Biochem. Biophys. 2016, 469, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Evangelidou, M.; Karamita, M.; Vamvakas, S.S.; Szymkowski, D.E.; Probert, L. Altered expression of oligodendrocyte and neuronal marker genes predicts the clinical onset of autoimmune encephalomyelitis and indicates the effectiveness of multiple sclerosis-directed therapeutics. J. Immunol. 2014, 192, 4122–4133. [Google Scholar] [CrossRef] [PubMed]
- Solga, A.C.; Pong, W.W.; Walker, J.; Wylie, T.; Magrini, V.; Apicelli, A.J.; Griffith, M.; Griffith, O.L.; Kohsaka, S.; Wu, G.F.; et al. Rna-sequencing reveals oligodendrocyte and neuronal transcripts in microglia relevant to central nervous system disease. Glia 2015, 63, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, G.; Chen, X.; Li, C.; Wang, L.; Liu, Y.; Han, D.; Liu, H.; Hou, X.; Zhang, W.; et al. Mir-300 promotes self-renewal and inhibits the differentiation of glioma stem-like cells. J. Mol. Neurosci. 2014, 53, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Z.; Li, X.; Liu, H. Microrna-365 inhibits growth, invasion and metastasis of malignant melanoma by targeting nrp1 expression. Int. J. Clin. Exp. Pathol. 2015, 8, 4913–4922. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Li, R.; Wang, Y.Y.; Shi, Y.; Luan, W.K.; Tao, T.; Zhang, J.X.; Xu, Y.C.; You, Y.P. Mir-1224–5p acts as a tumor suppressor by targeting creb1 in malignant gliomas. Mol. Cell. Biochem. 2015, 403, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Belogurov, A., Jr.; Kuzina, E.; Kudriaeva, A.; Kononikhin, A.; Kovalchuk, S.; Surina, Y.; Smirnov, I.; Lomakin, Y.; Bacheva, A.; Stepanov, A.; et al. Ubiquitin-independent proteosomal degradation of myelin basic protein contributes to development of neurodegenerative autoimmunity. FASEB J. 2015, 29, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Belogurov, A., Jr.; Kudriaeva, A.; Kuzina, E.; Smirnov, I.; Bobik, T.; Ponomarenko, N.; Kravtsova-Ivantsiv, Y.; Ciechanover, A.; Gabibov, A. Multiple sclerosis autoantigen myelin basic protein escapes control by ubiquitination during proteasomal degradation. J. Biol. Chem. 2014, 289, 17758–17766. [Google Scholar] [CrossRef] [PubMed]
- Kuzina, E.; Kudriaeva, A.; Smirnov, I.; Dubina, M.V.; Gabibov, A.; Belogurov, A., Jr. Glatiramer acetate and nanny proteins restrict access of the multiple sclerosis autoantigen myelin basic protein to the 26s proteasome. Biomed. Res. Int. 2014, 2014, 926394. [Google Scholar] [CrossRef] [PubMed]
- Kuzina, E.S.; Chernolovskaya, E.L.; Kudriaeva, A.A.; Zenkova, M.A.; Knorre, V.D.; Surina, E.A.; Ponomarenko, N.A.; Bobik, T.V.; Smirnov, I.V.; Bacheva, A.V.; et al. Immunoproteasome enhances intracellular proteolysis of myelin basic protein. Dokl. Biochem. Biophys. 2013, 453, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Tewari, M.K.; Sinnathamby, G.; Rajagopal, D.; Eisenlohr, L.C. A cytosolic pathway for mhc class ii-restricted antigen processing that is proteasome and tap dependent. Nat. Immunol. 2005, 6, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Fabre, B.; Lambour, T.; Garrigues, L.; Amalric, F.; Vigneron, N.; Menneteau, T.; Stella, A.; Monsarrat, B.; Van den Eynde, B.; Burlet-Schiltz, O.; et al. Deciphering preferential interactions within supramolecular protein complexes: The proteasome case. Mol. Syst. Biol. 2015, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Groettrup, M.; Soza, A.; Eggers, M.; Kuehn, L.; Dick, T.P.; Schild, H.; Rammensee, H.G.; Koszinowski, U.H.; Kloetzel, P.M. A role for the proteasome regulator pa28alpha in antigen presentation. Nature 1996, 381, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Belogurov, A., Jr.; (Shemyakin-Ovchinnikov Institute of bioorganic chemistry, Moscow, Russia). Personal communication, 2016.
- McCarthy, K.D.; de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.A.; Spinnenhirn, V.; Schmidtke, G.; Basler, M.; Groettrup, M.; Goldberg, A.L. Immuno- and constitutive proteasomes do not differ in their abilities to degrade ubiquitinated proteins. Cell 2013, 152, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Maltseva, D.V.; Khaustova, N.A.; Fedotov, N.N.; Matveeva, E.O.; Lebedev, A.E.; Shkurnikov, M.U.; Galatenko, V.V.; Schumacher, U.; Tonevitsky, A.G. High-throughput identification of reference genes for research and clinical rt-qpcr analysis of breast cancer samples. J. Clin. Bioinform. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Krainova, N.A.; Khaustova, N.A.; Makeeva, D.S.; Fedotov, N.N.; Gudim, E.A.; Ryabenko, E.A.; Shkurnikov, M.U.; Galatenko, V.V.; Sakharov, D.A.; Maltseva, D.V. Evaluation of potential reference genes for qRT-PCR data normalization in hela cells. Appl. Biochem. Microbiol. 2013, 49, 743–749. [Google Scholar] [CrossRef]
- Oliveira-Ferrer, L.; Rossler, K.; Haustein, V.; Schroder, C.; Wicklein, D.; Maltseva, D.; Khaustova, N.; Samatov, T.; Tonevitsky, A.; Mahner, S.; et al. C-fos suppresses ovarian cancer progression by changing adhesion. Br. J. Cancer 2014, 110, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Sakharov, D.A.; Maltseva, D.V.; Riabenko, E.A.; Shkurnikov, M.U.; Northoff, H.; Tonevitsky, A.G.; Grigoriev, A.I. Passing the anaerobic threshold is associated with substantial changes in the gene expression profile in white blood cells. Eur. J. Appl. Physiol. 2012, 112, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yan, Y.; Li, X.; Li, K.; Ciric, B.; Yang, J.; Zhang, Y.; Wu, S.; Xu, H.; Chen, W.; et al. Silencing ifn-gamma binding/signaling in astrocytes versus microglia leads to opposite effects on central nervous system autoimmunity. J. Immunol. 2015, 194, 4251–4264. [Google Scholar] [CrossRef] [PubMed]
- Hidano, S.; Randall, L.M.; Dawson, L.; Dietrich, H.K.; Konradt, C.; Klover, P.J.; John, B.; Harris, T.H.; Fang, Q.; Turek, B.; et al. Stat1 signaling in astrocytes is essential for control of infection in the central nervous system. mBio 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aki, M.; Shimbara, N.; Takashina, M.; Akiyama, K.; Kagawa, S.; Tamura, T.; Tanahashi, N.; Yoshimura, T.; Tanaka, K.; Ichihara, A. Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J. Biochem. 1994, 115, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Namiki, S.; Nakamura, T.; Oshima, S.; Yamazaki, M.; Sekine, Y.; Tsuchiya, K.; Okamoto, R.; Kanai, T.; Watanabe, M. Irf-1 mediates upregulation of lmp7 by ifn-gamma and concerted expression of immunosubunits of the proteasome. FEBS Lett. 2005, 579, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Brucet, M.; Marques, L.; Sebastian, C.; Lloberas, J.; Celada, A. Regulation of murine tap1 and lmp2 genes in macrophages by interferon gamma is mediated by stat1 and irf-1. Genes Immun. 2004, 5, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Meissner, T.B.; Li, A.; Biswas, A.; Lee, K.H.; Liu, Y.J.; Bayir, E.; Iliopoulos, D.; van den Elsen, P.J.; Kobayashi, K.S. Nlr family member nlrc5 is a transcriptional regulator of mhc class i genes. Proc. Natl. Acad. Sci. USA 2010, 107, 13794–13799. [Google Scholar] [CrossRef] [PubMed]
- Steimle, V.; Siegrist, C.A.; Mottet, A.; Lisowska-Grospierre, B.; Mach, B. Regulation of mhc class ii expression by interferon-gamma mediated by the transactivator gene ciita. Science 1994, 265, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.S.; van den Elsen, P.J. Nlrc5: A key regulator of mhc class i-dependent immune responses. Nat. Rev. Immunol. 2012, 12, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Reith, W.; LeibundGut-Landmann, S.; Waldburger, J.M. Regulation of mhc class II gene expression by the class ii transactivator. Nat. Rev. Immunol. 2005, 5, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.S.; Tani, M.; Ransohoff, R.M.; Wysocka, M.; Hilliard, B.; Fujioka, T.; Murphy, S.; Tighe, P.J.; Das Sarma, J.; Trinchieri, G.; et al. Astrocytes as antigen-presenting cells: Expression of il-12/il-23. J. Neurochem. 2005, 95, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Schwab, N.; Bien, C.G.; Waschbisch, A.; Becker, A.; Vince, G.H.; Dornmair, K.; Wiendl, H. Cd8+ T-cell clones dominate brain infiltrates in rasmussen encephalitis and persist in the periphery. Brain 2009, 132, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound benztropine are available from the authors. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudriaeva, A.; Galatenko, V.V.; Maltseva, D.V.; Khaustova, N.A.; Kuzina, E.; Tonevitsky, A.G.; Gabibov, A.; Belogurov, A. The Transcriptome of Type I Murine Astrocytes under Interferon-Gamma Exposure and Remyelination Stimulus. Molecules 2017, 22, 808. https://doi.org/10.3390/molecules22050808
Kudriaeva A, Galatenko VV, Maltseva DV, Khaustova NA, Kuzina E, Tonevitsky AG, Gabibov A, Belogurov A. The Transcriptome of Type I Murine Astrocytes under Interferon-Gamma Exposure and Remyelination Stimulus. Molecules. 2017; 22(5):808. https://doi.org/10.3390/molecules22050808
Chicago/Turabian StyleKudriaeva, Anna, Vladimir V. Galatenko, Diana V. Maltseva, Nadezhda A. Khaustova, Ekaterina Kuzina, Alexander G. Tonevitsky, Alexander Gabibov, and Alexey Belogurov. 2017. "The Transcriptome of Type I Murine Astrocytes under Interferon-Gamma Exposure and Remyelination Stimulus" Molecules 22, no. 5: 808. https://doi.org/10.3390/molecules22050808
APA StyleKudriaeva, A., Galatenko, V. V., Maltseva, D. V., Khaustova, N. A., Kuzina, E., Tonevitsky, A. G., Gabibov, A., & Belogurov, A. (2017). The Transcriptome of Type I Murine Astrocytes under Interferon-Gamma Exposure and Remyelination Stimulus. Molecules, 22(5), 808. https://doi.org/10.3390/molecules22050808