New Mild and Simple Approach to Isothiocyanates: A Class of Potent Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
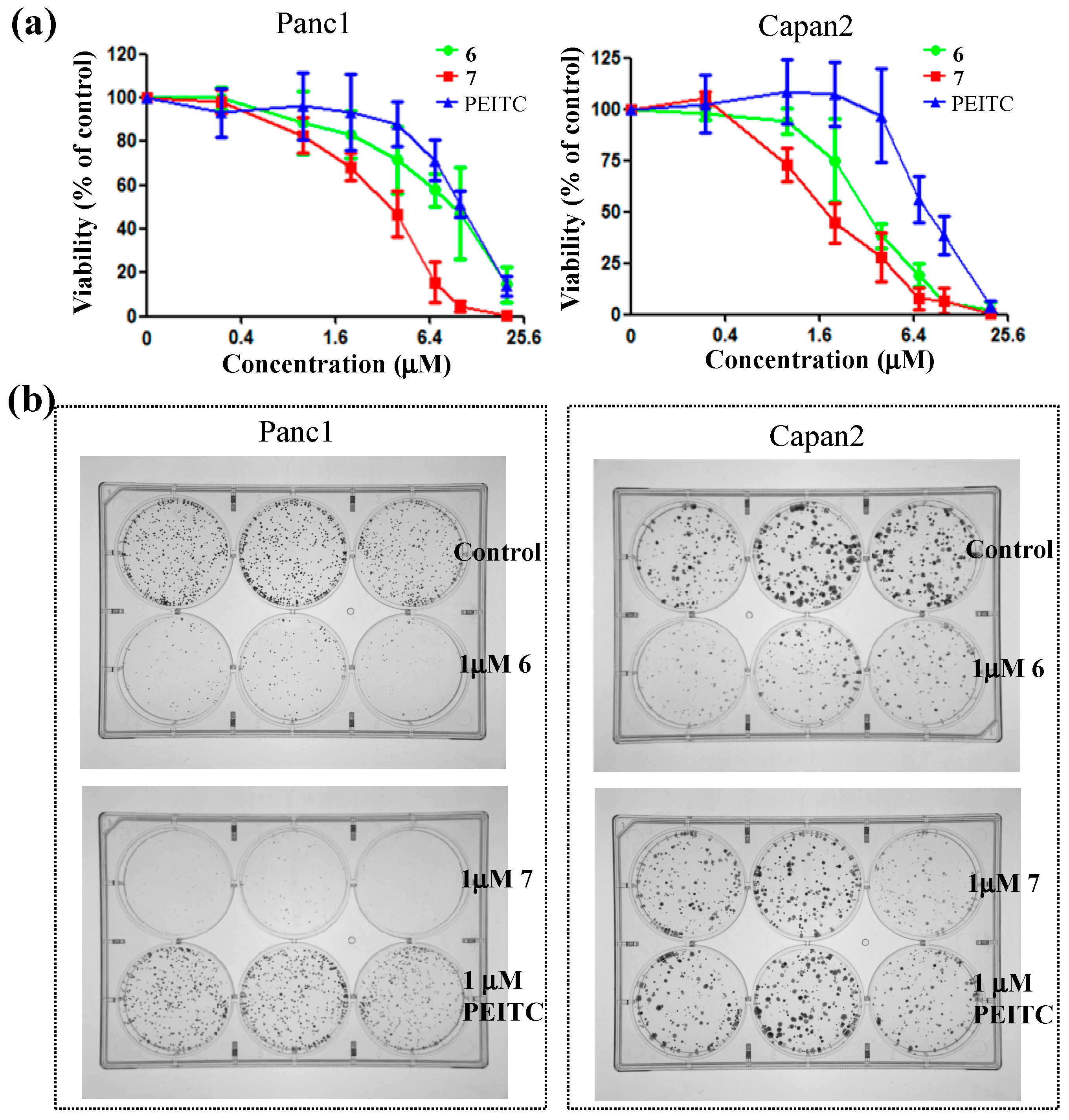
2.2. Biology
3. Materials and Methods
3.1. General Procedure Exemplified by the Synthesis of PEITC
3.2. Characterization of Synthetic Isothiocyanates
3.3. Biological Cellular Assay
3.3.1. Cell Culture
3.3.2. MTS Assay
3.3.3. Colony Formation
3.3.4. Apoptosis and ROS Measurement
3.3.5. GSH Levels
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferguson, L.R.; Philpott, M. Cancer prevention by dietary bioactive components that target the immune response. Curr. Cancer Drug Targets 2007, 7, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Khor, T.O.; Huang, M.T.; Kong, A.N. Differential in vivo mechanism of chemoprevention of tumor formation in azoxymethane/dextran sodium sulfate mice by PEITC and DBM. Carcinogenesis 2010, 31, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Kim, B.R.; Chen, C.; Hebbar, V.; Kong, A.N. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Powolny, A.A.; Moura, M.B.; Kelley, E.E.; Bommareddy, A.; Kim, S.H.; Hahm, E.R.; Normolle, D.; Van Houten, B.; Singh, S.V. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J. Biol. Chem. 2010, 285, 26558–26569. [Google Scholar] [CrossRef] [PubMed]
- Runarsdottir, A.; Mannervik, B. A novel quasi-species of glutathione transferase with high activity towards naturally occurring isothiocyanates evolves from promiscuous low-activity variants. J. Mol. Biol. 2010, 401, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Syed Alwi, S.S.; Cavell, B.E.; Donlevy, A.; Packham, G. Differential induction of apoptosis in human breast cancer cell lines by phenethyl isothiocyanate, a glutathione depleting agent. Cell Stress Chaperones 2012, 17, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, A.; Lemm, E.; Wilmore, S.; Cavell, B.E.; Valle-Argos, B.; Krysov, S.; Hidalgo, M.S.; Leonard, E.; Willis, A.E.; Forconi, F.; et al. PEITC-mediated inhibition of mRNA translation is associated with both inhibition of mTORC1 and increased eIF2alpha phosphorylation in established cell lines and primary human leukemia cells. Oncotarget 2016, 7, 74807–74819. [Google Scholar] [PubMed]
- Cavell, B.E.; Syed Alwi, S.S.; Donlevy, A.M.; Proud, C.G.; Packham, G. Natural product-derived antitumor compound phenethyl isothiocyanate inhibits mTORC1 activity via TSC2. J. Nat. Prod. 2012, 75, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Di Pasqua, A.J.; Govind, S.; McCracken, E.; Hong, C.; Mi, L.; Mao, Y.; Wu, J.Y.; Tomita, Y.; Woodrick, J.C.; et al. Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships. J. Med. Chem. 2011, 54, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, Z.; Hu, Y.; Huang, P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by beta-phenethyl isothiocyanate: mechanisms for anti-leukemia activity. Antioxid. Redox Signal. 2011, 15, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Zhang, H.; Zhang, W.; Feng, L.; Du, M.; Zhou, Y.; Chen, Z.; Pelicano, H.; Plunkett, W.; Wierda, W.G.; et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood 2008, 112, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J.; et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Mays, J.R.; Weller Roska, R.L.; Sarfaraz, S.; Mukhtar, H.; Rajski, S.R. Identification, synthesis, and enzymology of non-natural glucosinolate chemopreventive candidates. ChemBioChem 2008, 9, 729–747. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Zwanenburg, B.; Chittenden, G.J.; Verhagen, H. Synthesis of isothiocyanate-derived mercapturic acids. Eur. J. Med. Chem. 2003, 38, 729–737. [Google Scholar] [CrossRef]
- Berglund, M.; Dalence-Guzman, M.F.; Skogvall, S.; Sterner, O. SAR studies of capsazepinoid bronchodilators 3: The thiourea part (coupling region) and the 2-(4-chlorophenyl)ethyl moiety (C-region). Bioorg. Med. Chem. 2008, 16, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Tsogoeva, S.B.; Hateley, M.J.; Yalalov, D.A.; Meindl, K.; Weckbecker, C.; Huthmacher, K. Thiourea-based non-nucleoside inhibitors of HIV reverse transcriptase as bifunctional organocatalysts in the asymmetric Strecker synthesis. Bioorg. Med. Chem. 2005, 13, 5680–5685. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Ishikawa, T. 2-Chloro-1, 3-dimethylimidazolinium chloride. 2. Its application to the construction of heterocycles through dehydration reactions. J. Org. Chem. 1999, 64, 6989–6992. [Google Scholar] [CrossRef]
- Munch, H.; Hansen, J.S.; Pittelkow, M.; Christensen, J.B.; Boas, U. A new efficient synthesis of isothiocyanates from amines using di-tert-butyl dicarbonate. Tetrahedron Lett. 2008, 49, 3117–3119. [Google Scholar] [CrossRef]
- Wong, R.; Dolman, S.J. Isothiocyanates from tosyl chloride mediated decomposition of in situ generated dithiocarbamic acid salts. J. Org. Chem. 2007, 72, 3969–3971. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Lowenfels, A.B. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int. J. Epidemiol. 2015, 44, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.K.; Wu, M.S.; Kakugawa, Y.; Kim, J.J.; Yeoh, K.G.; Goh, K.L.; Wu, K.C.; Wu, D.C.; Sollano, J.; Kachintorn, U.; et al. Screening for gastric cancer in Asia: Current evidence and practice. Lancet Oncol. 2008, 9, 279–287. [Google Scholar] [CrossRef]
- Zhang, E.B.; Han, L.; Yin, D.D.; Kong, R.; De, W.; Chen, J. C-Myc-induced, long, noncoding H19 affects cell proliferation and predicts a poor prognosis in patients with gastric cancer. Med. Oncol. 2014, 31, 914. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Cidlowski, J.A. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 2009, 16, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.T.; Yen, Y.W.; Lo, C.L. Reactive oxygen species and glutathione dual redox-responsive micelles for selective cytotoxicity of cancer. Biomaterials 2015, 61, 150–161. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 6, 7 are available from the authors. |




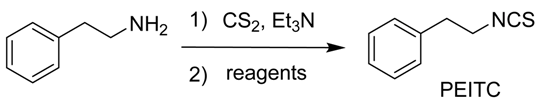
| Entry | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Reagent | AcCl | TsCl | SiMe2Cl2 | BzCl | SO2Cl2 |
| Yield | 94% | 70% | 79% | 42% | 80% |
| Compound | IC50 (μM) | |
|---|---|---|
| Panc1 | HGC27 | |
| PEITC | 10.80 ± 0.25 | 2.95 ± 0.41 |
| 1 | 15.80 ± 1.06 | 8.79 ± 0.72 |
| 2 | 10.87 ± 0.47 | 1.49 ± 0.06 |
| 3 | 14.24 ± 0.50 | 2.73 ± 0.29 |
| 4 | 6.12 ± 0.13 | 3.44 ± 0.23 |
| 5 | 8.05 ± 0.36 | 2.67 ± 0.37 |
| 6 | 4.55 ± 0.32 | 1.09 ± 0.07 |
| 7 | 2.04 ± 0.21 | 0.46 ± 0.02 |
| 8 | 5.78 ± 0.56 | 0.74 ± 0.01 |
| 9 | 3.52 ± 0.11 | 0.84 ± 0.06 |
| 10 | 2.68 ± 0.21 | 0.93 ± 0.41 |
| 11 | 5.08 ± 0.02 | 1.47 ± 0.10 |
| 12 | 8.92 ± 0.43 | 0.72 ± 0.21 |
| 13 | 5.65 ± 0.16 | 0.99 ± 0.15 |
| 14 | 2.25 ± 0.21 | 0.62 ± 0.04 |
| 15 | 3.76 ± 0.05 | 1.68 ± 0.00 |
| 16 | 6.24 ± 0.13 | 2.37 ± 0.43 |
| 17 | 4.70 ± 0.27 | 0.77 ± 0.18 |
| 18 | 2.78 ± 0.72 | 0.34 ± 0.00 |
| 19 | 2.14 ± 0.79 | 0.72 ± 0.08 |
| 20 | 4.06 ± 0.57 | 0.82 ± 0.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.; Wang, J.; Li, X.; Lu, W.; Yang, J.; Hu, Y.; Huang, P.; Wen, S. New Mild and Simple Approach to Isothiocyanates: A Class of Potent Anticancer Agents. Molecules 2017, 22, 773. https://doi.org/10.3390/molecules22060773
Luo B, Wang J, Li X, Lu W, Yang J, Hu Y, Huang P, Wen S. New Mild and Simple Approach to Isothiocyanates: A Class of Potent Anticancer Agents. Molecules. 2017; 22(6):773. https://doi.org/10.3390/molecules22060773
Chicago/Turabian StyleLuo, Bingling, Jiankang Wang, Xiaobing Li, Wenhua Lu, Jing Yang, Yumin Hu, Peng Huang, and Shijun Wen. 2017. "New Mild and Simple Approach to Isothiocyanates: A Class of Potent Anticancer Agents" Molecules 22, no. 6: 773. https://doi.org/10.3390/molecules22060773
APA StyleLuo, B., Wang, J., Li, X., Lu, W., Yang, J., Hu, Y., Huang, P., & Wen, S. (2017). New Mild and Simple Approach to Isothiocyanates: A Class of Potent Anticancer Agents. Molecules, 22(6), 773. https://doi.org/10.3390/molecules22060773








