Abstract
Hydroxybenzylidene hydrazines exhibit a wide spectrum of biological activities. Here, we report synthesis and free radical scavenging activity of nine new N-(hydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazines. The chemical structures of these compounds were confirmed by 1H-NMR, 13C-NMR, 19F-NMR, IR spectroscopy, LC-MS, and elemental analysis. The prepared compounds were tested for their activity to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH), galvinoxyl radical (GOR), and 2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulphonic acid (ABTS) radicals. The free radical scavenging activity expressed as SC50 values of these compounds varied in a wide range, from a strong to no radical scavenging effect. The most effective radical scavengers were hydroxybenzylidene hydrazines containing three hydroxyl groups in the benzylidene part of their molecules. The prepared compounds were also tested for their activity to inhibit photosynthetic electron transport in spinach chloroplasts. IC50 values of these compounds varied in wide range, from an intermediate to no inhibitory effect.
1. Introduction
N-Hydroxybenzylidene hydrazines, also known as hydrazones, are N-arylmethylidene-N′-arylhydrazines with a diazamethylidene group C=N-NH [1,2]. The fact that N-arylmethylidene-N′-arylhydrazines represent an important class of compounds for new drug development motivates researchers to synthesize and test new N-arylmethylidene-N′-arylhydrazines. These compounds can be prepared from the corresponding aromatic aldehydes and substituted arylhydrazines in alcohol (ethanol, methanol), acetic acid, or another solvents [1,2]. Synthesis without solvents using acidic ionic liquids such as choline chloride and oxalic acid has also been reported [3]. The combination of diazamethylene group with other functional groups leads to compounds with interesting physical and chemical characteristics [4].
The synthesis of novel N-arylmethylidene-N′-arylhydrazines and their derivatives is of great interest because of their potential use in the biopharmaceutic industry. These compounds possess various biological and pharmacological properties, including antimicrobial, analgesic, antifungal, anti-tubercular, antiviral, anticancer, antimalarial, antihelmintic, anti-trypanosomal, and antischistosomiasis properties. In addition, N-arylmethylidene-N′-arylhydrazines are used as pigments, dyes, catalysts, ligands in organometallic complexes and polymer stabilizers [5,6,7,8,9,10,11,12]. N-arylmethylidene-N′-arylhydrazines are also important for the synthesis of heterocyclic compounds such as indoles and pyrazoles [13,14]. Derivatives of benzylidene hydrazine are potent inhibitors of fungal growth with little mammalian cell toxicity, making them promising new targets for future therapeutic development [1]. Several derivatives of N-nitrobenzylidene-N′-phenylhydrazines exhibit amoebicidal activity with an IC50 of 0.84 μM, which represents a sevenfold increase in cell growth inhibition potency with respect to metronidazole (IC50 = 6.3 μM) [12]. Several novel 2,4-dinitrophenylhydrazone betulinic acid derivatives showed significant cytotoxicity and selectivity against some tumor cell lines [15]. Due to their strong chemical stability, N-arylmethylidene-N′-arylhydrazines are also an attractive material in optoelectronics technologies and development of potential chemosensors, optical switching devices, and organic light emission devices (OLEDs) [16]. However, N-arylmethylidene-N′-arylhydrazines also show adverse effects. Some N-arylmethylidene-N′-arylhydrazines induce DNA fragmentation [17] and damage photosynthesis in chloroplasts [18,19,20,21].
The main aim of this work was to synthesize new hydroxybenzylidene hydrazines and determine their free radical scavenging activity. We prepared nine new N-(hydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazines with OH groups at different positions of the benzene ring and analyzed their ability to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH), galvinoxyl radicals (GOR), and 2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulphonic acid (ABTS).
2. Results and Discussion
2.1. Chemistry
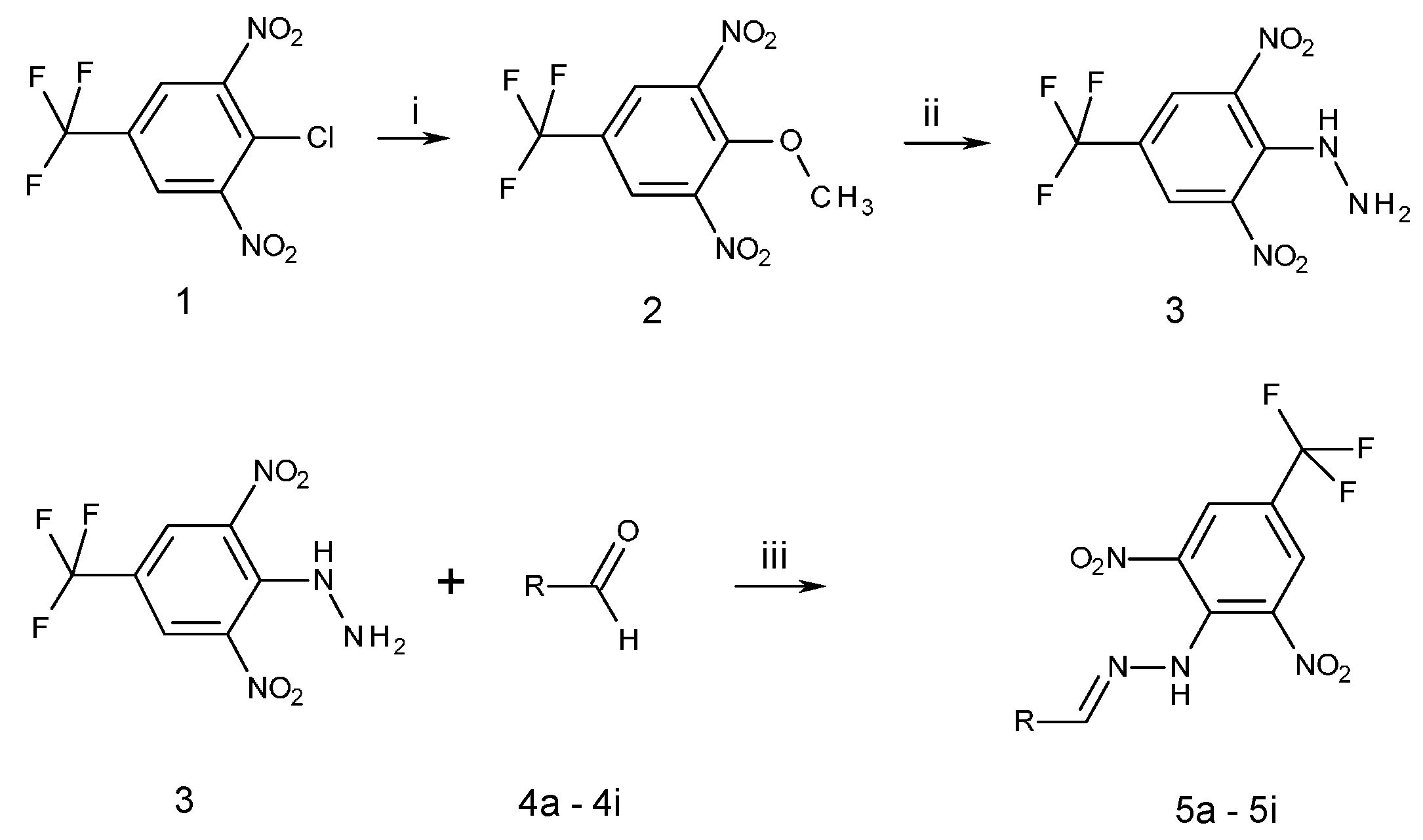
All compounds prepared in this study (5a–5i) contain the 2,4-dinitro-4-(trifluoromethyl)phenyl group and an additional hydroxyphenyl group (5a, R = 4-hydroxyphenyl, 5b, R = 2,3-dihydroxyphenyl, 5c, R = 2,4-dihydroxyphenyl, 5d, R = 2,5-dihydroxyphenyl, 5e, R = 3,5-dihydroxyphenyl, 5f, R = 2,3,4-trihydroxyphenyl, 5g, R = 2,4,6-trihydroxyphenyl, 5h, R = 3,4,5-trihydroxyphenyl, and 5i, R = phenyl) (Scheme 1).
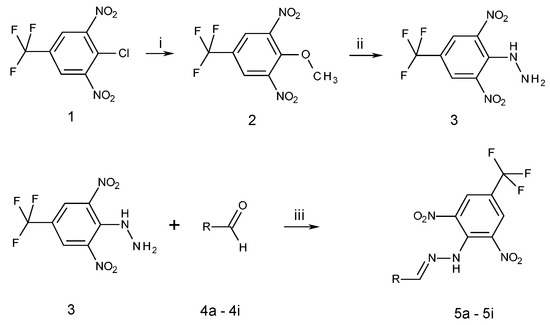
Scheme 1.
Synthesis of 2,6-dinitro-4-(trifluoromethyl)phenylhydrazine 3 and N-(hydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazines 5a–5i. 5a R = 4-hydroxyphenyl, 5b R = 2,3-dihydroxyphenyl, 5c R = 2,4-dihydroxyphenyl, 5d R = 2,5-dihydroxyphenyl, 5e R = 3,5-dihydroxyphenyl, 5f R = 2,3,4-trihydroxyphenyl, 5g R = 2,4,6-trihydroxyphenyl, 5h R = 3,4,5-trihydroxyphenyl, 5i R = phenyl. Reactants and reaction condition: (i) H3C-ONa, methanol, room temperature, 1 h; (ii) H2N-NH2·H2O, EtOH, 0 °C, 1 h; (iii) EtOH, trifluoroacetic acid (TFA), room temperature, 3 h. Structure of all newly prepared compounds was confirmed by 1H-NMR, 13C-NMR, 19F-NMR, IR spectroscopy, and elemental analysis.
The starting compounds for the synthesis of hydroxybenzylidene hydrazines 5a–5i were 2,6-dinitro-4-(trifluoromethyl)phenylhydrazine 3 and aromatic aldehydes 4a–4i (Scheme 1). The starting compound for the preparation of 2-methoxy-1,3-dinitro-5-(trifluoromethyl)benzene 2 (Scheme 1) was 2-chloro-1,3-dinitro-4-(trifluoromethyl)benzene 1. The yield of the compound 2 was 76%, M.p. 61 °C [22].
For the synthesis of compounds 5a–5i, we used trifluoroacetic acid (TFA) as acidic catalyst and ethanol as solvent. The reaction time was 3–4 h, reaction temperatures were 20–25 °C, and yields were 70–85%. The purity of prepared compounds and the course of reactions were monitored by TLC. The crude hydrazines 5a–5i were purified by column chromatography on silica gel in hexane/ethylacetate (4:1) as the mobile phase. All prepared hydroxybenzylidene hydrazines 5a–5i as well as their solutions in organic solvents were dark red. The dark red color changed to dark blue upon the increase in pH, suggesting that these compounds are sensitive to pH. The melting points of these compounds are relatively high (226–280 °C). The chemical structures of prepared compounds were confirmed by 1H-NMR, 13C-NMR, 19F-NMR, IR spectroscopy, LC-MS and elemental analysis. Elemental analyses agreed with theoretical values (±0.3). The IR spectra revealed several characteristic absorption bands. Two absorption bands were observed in the region stretching NO2 vibration. The more intense band appearing at higher wave numbers (1538–1503 cm−1) corresponds to assymetric NO2 vibrations, and the less intense one, appearing at a lower wave number (1279–1257 cm−1), corresponds to symmetric vibrations. The absorption band at 3277–3257 cm−1 corresponds to stretching N–H vibrations. The wave number of this absorption band is affected by the mesomeric effect of OH groups on the benzene ring. The absorption band at 1619–1616 cm−1 corresponds to stretching vibrations in the C=N group. The presence of CF3 group in molecules was confirmed by 19F-13C splitting to quartet in 13C NMR spectra of corresponding derivatives [4′ (115.82–116.71 ppm, 2J = 35.1–35.4 Hz); 3′,5′ (127.189–127.72 ppm, 3J = 3.2–3.5 Hz); CF3 (122.67–123.20 ppm, 1J = 271.3–271.6 Hz)].
The double bond between C and N in C=N–NH– group of benzylidene hydrazines allows for the formation of two stereoisomers, namely (E) and (Z). The stereoisomerism of these compounds has not been studied in detail, but we predict that (E) is the more abundant form [2,23].
2.2. Free Radical Scavenging Activity Assay
SC50 values for scavenging DPPH, GOR, and ABTS radicals are shown in the Table 1. These results suggest that the majority of studied hydroxybenzylidene hydrazines exhibit free radical scavenging activity. Almost all studied benzylidene hydrazines (with the exception of compound 5i) scavenged ABTS radical in a water solution. The most effective scavengers of ABTS radicals were compounds 5b and 5e with two OH groups in positions 2, 3 and 3, 5, respectively. Hydroxybenzylidene hydrazines with three hydroxyl groups (5b, 5f, 5h and 5g) also scavenged ABTS radicals. The most effective scavengers of DPPH radicals were hydroxybenzylidene hydrazines with three OH groups (5h and 5f). The molecules with two OH groups (5b, 5c, 5g) were weaker scavengers of DPPH radicals. Hydroxybenzylidene hydrazine with one OH group (5a) exhibited very weak scavenger activity, and the compound with OH groups in positions 3 and 5 (5e) as well as the compound without OH group (5i) did not scavenge DPPH radicals.

Table 1.
Antiradical and PET inhibition activities of studied hydroxybenzilidene hydrazines expressed as SC50 and IC50 values, respectively.
The compound with three OH groups (5f) in positions 2, 3, and 4 was the most effective scavenger of GOR radicals. The compounds 5b, 5d, 5g, and 5h exhibited moderate scavenger activity, and the compound 5a (with one OH group) exhibited very low activity to scavenge GOR radicals. Similarly as observed with DPPH radicals, the compounds 5e and 5i showed no scavenging of GOR.
Taken together, we conclude that molecules with three hydroxyl groups (5b, 5f, and 5h) exhibited high free radical scavenging activities. The most effective compound was the compound 5f, which contains OH groups in positions 2, 3, and 4. Interestingly, the compound with no hydroxyl group (5i) showed no scavenging activity. Some of the studied compounds (5f and 5h) exhibited higher free radical scavenging activity than that reported for ascorbic acid (SC50 = 12.55 μmol/dm3) [24], resveratrol (SC50 = 26.37 μmol/dm3) [25], and esculetin (SC50 = 8.64 μmol/dm3) [26]. We speculate that the ability to release hydrogen atom or proton from hydroxyl groups of the hydroxybenzylidene hydrazine molecule contributes to the mechanism of radical scavenging of the studied hydroxybenzylidene hydrazines.
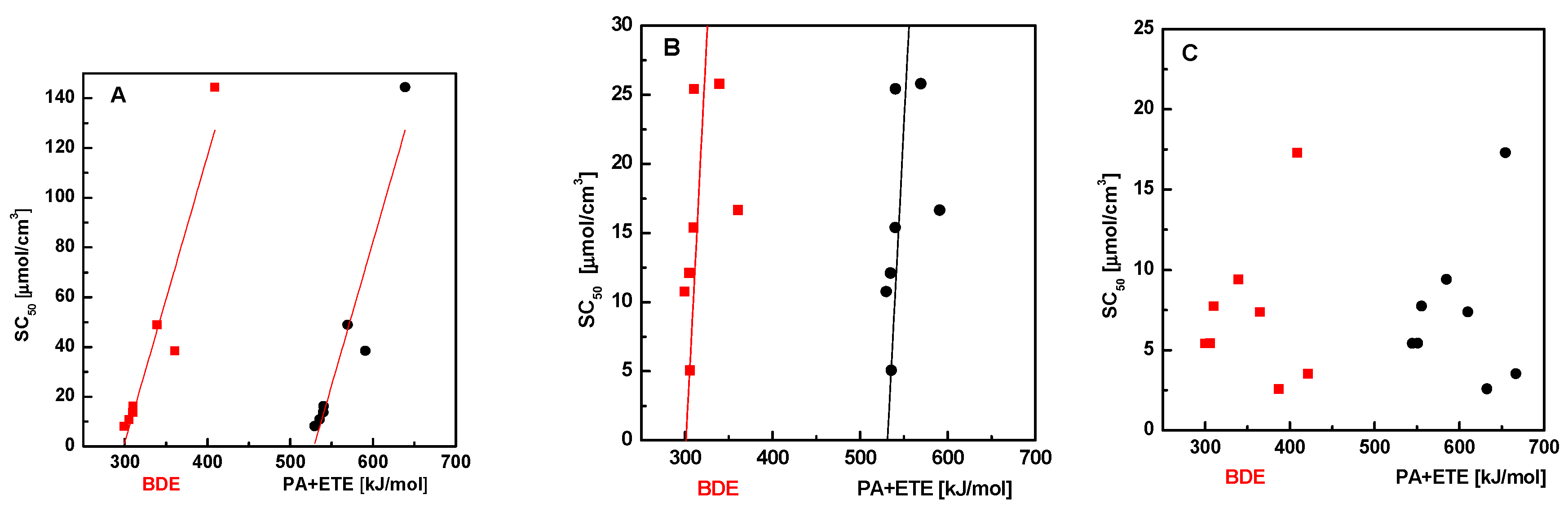
Next, we tried to find a possible correlation between free radical scavenging activity and energy necessary for releasing of hydrogen atom or proton. We used the method PM6 (see the Experimental Section) to calculate the energy associated with the release of hydrogen atom (bond dissociation enthalpy, BDE), the proton relaxation (proton dissociation enthalpy, PDE), release of electron (ionization potential, IP), and the energy associated with the combined transfer of the electron and proton (ETE and PA, respectively) for the studied benzylidene hydrazines. The values of these energies in methanol or in water are presented in Tables S1–S8. Figure 1 shows the dependence of SC50 of DPPH, GOR, and ABTS scavenging on the sum of PA + ETE values and BDE, respectively. The results presented in the Figure 1A,B suggest that the ability of scavenging of DPPH and GOR radicals at higher BDE and PE + ETE enthalpy decreases—the dependence is almost linear (the square deviation r2 = 0.87 for DPPH and r2 = 0.70 for GOR). This tendency is confirmed by the fact that the inactive substances (5a and 5e) have the greatest values of BDE and PA + ETE (Table 1). On the other hand, the dependence of the scavenging of ABTS radicals on BDE or PA + ETE shows no dependence (Figure 1C).
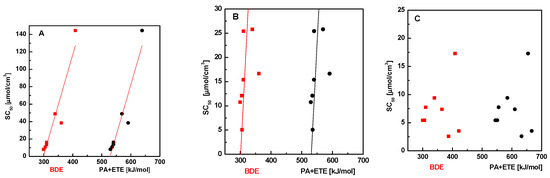
Figure 1.
Dependence of SC50 of DPPH (A), GOR (B), and ABTS (C) scavenging on BDE (red squares) or PA + ETE (black circles).
2.3. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
The prepared compounds were also tested for their activity to inhibit PET in spinach chloroplasts. IC50 values of these compounds varied in a wide range, from an intermediate (14.7 μmol/dm3) to no inhibitory effect (Table 1). The most effective were compounds 5c (IC50 = 14.7 μmol/dm3) and 5b (IC50 = 42.8 μmol/dm3). These activities are relatively low as compared to the values reported for the classical herbicide diuron (3-(3,4-dichlorophenyl)-1,1-dimethylurea; DCMU) with an IC50 of 1.9 μmol/dm3 [27] and are unlikely to be of industrial interest.
3. Materials and Methods
3.1. General Information
2-Chloro-1,3-dinitro-4-(trifluoromethyl)benzene 1 (Scheme 1), galvinoxyl free radical (GOR), and organic solvents were purchased from Alfa Aesar (Ward Hill, MA, USA) and used without further purification. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Methyl alcohol p.a., TRIS, MgCl2, saccharose and dimethylsulfoxide p.a. (DMSO) were purchased from Centralchem (Bratislava, Slovakia).
Melting points were determined on a Boetius apparatus and are uncorrected. IR spectra were obtained on a NICOLET iS50 FT-IR spectrophotometer using an ATR technique in the region 4000–400 cm−1. Elemental analyses were obtained on an Elemental Analyzer Carlo Erba CHNS-OEA 1108. NMR spectra were performed on a Spectrometer Varian VNMRS 300 MHz (300 MHz for 1H, 75 MHz for 13C, 282 MHz for 19F) and on a Spectrometer Varian VNMRS 600 MHz (600 MHz for 1H and 150 MHz for 13C) in DMSO-d6, with tetramethylsilane (TMS) as an internal standard. The purity of prepared compounds and the course of reactions were checked on Merck TLC Silica gel 60 F254 plates in ethyl acetate–n-hexane as the mobile phase. The numbering of atoms for the evaluation of NMR spectra of measured compounds 3 and 5a–5i is given in the formulae. Absorption spectra were recorded by a Genesis 6 spectrophotometer (Thermo–Scientific, Waltham, MA, USA). FTIR spectra (in solid phase) were recorded on a Nicolet 6700 spectrometer (Thermo–Scientific (Nicolet), Waltham, MA, USA) using the ATR technique.
MS spectra were recorded by LC-MS spectrometer consisting of an Agilent 1200 HPLC (Walbron, Germany), with an MSD 6110 MS detector (Agilent Technologies, Santa Clara, CA, USA).
3.2. Synthesis
3.2.1. Synthesis of 2,6-Dinitro-4-(Trifluoromethyl)Phenylhydrazine (3)
A solution of hydrazine monohydrate (0.75 g, 15 mmol) in anhydrous ethanol (5 mL) was added dropwise to a solution of 2-methoxy-1,3-dinitro-5-(trifluoromethyl)benzene 2 (3.2 g, 12 mmol) in anhydrous ethanol (17 mL) under an argon atmosphere. The reaction mixture was stirred at 0 °C for 1 h and monitored by TLC. The solvent was removed under vacuum, and crude product was purified by column chromatography on silica gel in hexane/ethylacetate (4:1) as the mobile phase. This resulted in 2.8 g (88%) of 2,6-dinitro-4-(trifluoromethyl)phenylhydrazine 3 (yellow solid), M.p. 125–126 °C. The previously published M.p. for this compound is 124 °C [23].
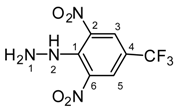
2,6-dinitro-4-(trifluoromethyl)phenylhydrazine (3). Yield 88%; red solid, M.p. 125–126 °C, Anal. Calcd. for C7H5F3N4O4 (266.14) C, 31.59; H, 1.89: N, 21.05. Found: C, 31.71; H, 1.74; N, 20.86%. IR: 3248, ʋ(N–H); 1636, ʋ(C=N); 1536, ʋas(NO2); 1263, ʋs(NO2); 1118, ʋ(CF3). 1H NMR (300 MHz, DMSO): δ = 9.65 (s, 1H, NH-2), 8.40 (s, 2H, H-3, H-5), 4.84 (s, 2H, NH-1). 13C NMR (75 MHz, DMSO): δ = 142.97, 136.75, 127.87 (q, J = 3.5 Hz), 123.32 (q, J = 271.0 Hz), 113.76 (q, J = 35.3 Hz); negative LC-MS m/z: 265.0 [M − H]− calc. for C7H4F3N4O4−, 265.018, found 265.0.
3.2.2. Synthesis of N-Hydroxybenzylidene-N′-[2,6-Dinitro-4-(Trifluoromethyl)]Phenylhydrazines (5a–5h) and N-(Benzylidene)-N′-[2,6-Dinitro-4-(Trifluoromethyl)]Phenylhydrazine (5i)
A solution of 2,6-dinitro-4(trifluoromethyl)phenylhydrazine 3 (1 mmol) in ethanol (3 mL) was added to a solution of aldehydes 4a–4i in ethanol (5 mL) and trifluoroacetic acid (0.5 mL). The reaction mixture was stirred for 3–4 h at room temperature in argon atmosphere and monitored by TLC. The reaction mixture was cooled to 0 °C, and the red solid product was filtered off, washed with ethanol/ether (1:5), and dried. The product was purified by column chromatography on silica gel in hexane/ethylacetate (3:1) as the mobile phase.
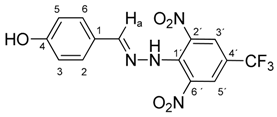
N-(4-Hydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazine (5a). Yield 72%; red solid, M.p. 246–248 °C, Anal. Calcd. for C7H9F3N4O5 (370.24) C, 45.42; H, 2.45: N, 15.13. Found: C, 45.30; H, 2.38; N, 15.10%. IR: 3507, ʋ(O–H); 3277, ʋ(N–H); 1636, ʋ(C=N); 1537, ʋas(NO2); 1261, ʋs(NO2); 1125, ʋ(CF3). 1H NMR (600 MHz, DMSO): δ = 11.46 (s, 1H, NH), 10.05 (s, 1H, OH), 8.53 (s, 2H, H-3′, H-5′), 8.41 (s, 1H, H-a), 7.39 (d, J = 8.6 Hz, 2H, H-2, H-6), 6.84 (d, J = 8.6 Hz, 2H, H-3, H-5). 13C NMR (151 MHz, DMSO): δ = 160.34, 149.53, 137.47, 136.16, 129.54, 127.64 (q, J = 3.3 Hz), 125.11, 123.17 (q, J = 271.5 Hz), 116.37 (q, J = 35.1 Hz), 116.32; negative LC-MS m/z: 369.0 [M − H]− calc. for C7H8F3N4O5−, 369.045, found 369.0.
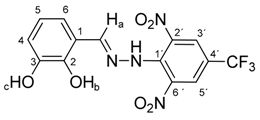
N-(2,3-Dihydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazine (5b). Yield 79%; red solid, M.p. 226–228 °C, Anal. Calcd. for C14H9F3N4O6 (386.24) C, 43.54; H, 2.35: N, 14.51. Found: C, 43.64; H, 2.39; N, 14.63%. IR: 3528, 3377 ʋ(O–H); 3274, ʋ(N–H); 1633, ʋ(C=N); 1537, ʋas(NO2); 1279, ʋs(NO2); 1125, ʋ(CF3). 1H NMR (600 MHz, DMSO): δ = 11.56 (s, 1H, NH), 9.63 (s, 1H, OH-b), 9.03 (s, 1H, OH-c), 8.79 (s, 1H, H-a), 8.51 (s, 2H, H-3′, H-5′), 6.93 (dd, J = 7.9, 1.5 Hz, 1H, H-6), 6.81 (dd, J = 7.9, 1.5 Hz, 1H, H-4), 6.65 (dd, J = 7.9 Hz, 1H, H-5). 13C NMR (151 MHz, DMSO): δ = 146.29, 146.19, 146.15, 137.71, 136.11, 127.63 (q, J = 3.4 Hz), 123.15 (q, J = 271.4 Hz), 121.24, 119.81, 117.36, 116.71 (q, J = 35.4 Hz), 116.44; negative LC-MS m/z: 385.0 [M − H]− calc. for C14H8F3N4O6−, 385.04, found 385.0.
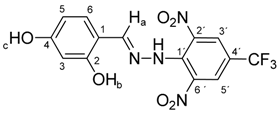
N-(2,4-Dihydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazine (5c). Yield 73%; red solid, M.p. 229–230 °C, Anal. Calcd. for C14H9F3N4O6 (386.24) C, 43.54; H, 2.35: N, 14.51. Found: C, 43.66; H, 2.24; N, 14.40%. IR: 3528, 3377 ʋ(O–H); 3274, ʋ(N–H); 1633, ʋ(C=N); 1537, ʋas(NO2); 1279, ʋs(NO2); 1125, ʋ(CF3). 1H NMR (600 MHz, DMSO): δ = 11.46 (s, 1H, NH), 9.94 (s, 1H, OH-b), 9.88 (s, 1H, OH-c), 8.63 (s, 1H, H-a), 8.48 (s, 2H, H-3′, H-5′), 7.27 (d, J = 8.6 Hz, 1H, H-6), 6.31 (d, J = 2.2 Hz, 1H, H-3), 6.28 (dd, J = 8.6, 2.2 Hz, 1H, H-5). 13C NMR (151 MHz, DMSO): δ = 161.69, 158.89, 146.59, 137.36, 136.07, 127.82, 127.67 (q, J = 3.2 Hz), 123.20 (q, J = 271.4 Hz), 115.93 (q, J = 35.3 Hz), 112.09, 108.66, 102.73; negative LC-MS m/z: 385.0 [M − H]− calc. for C14H8F3N4O6−, 385.04, found 385.0.
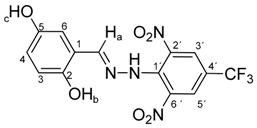
N-(2,5-Dihydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazine (5d). Yield 83%; red solid, M.p. 233–234 °C, Anal. Calcd. for C14H9F3N4O6 (386.24) C, 43.54; H, 2.35: N, 14.51. Found: C, 43.41; H, 2.42; N, 14.62%. IR: 3517, 3346 ʋ(O–H); 3257, ʋ(N–H); 1635, ʋ(C=N); 1536, ʋas(NO2); 1270, ʋs(NO2); 1129, ʋ(CF3). 1H NMR (600 MHz, DMSO): δ = 11.55 (s, 1H, NH), 9.40 (s, 1H, OH-b), 8.95 (s, 1H, OH-c), 8.73 (s, 1H, H-a), 8.54 (s, 2H, H-3′, H-5′), 6.85 (d, J = 2.1 Hz, 1H, H-6), 6.73 (s, 2H, H-4, H-3). 13C NMR (151 MHz, DMSO): δ = 150.42, 150.34, 146.27, 137.74, 136.10, 127.64 (q, J = 3.5 Hz), 123.15 (q, J = 271.6 Hz), 120.70, 120.21, 117.42, 116.71 (q, J = 35.3 Hz), 111.37; negative LC-MS m/z: 385.1 [M − H]− calc. for C14H8F3N4O6−, 385.04, found 385.1.
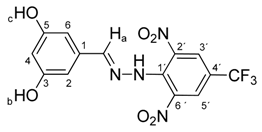
N-(3,5-Dihydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazine (5e). Yield 78%; red solid, M.p. 264–266 °C, Anal. Calcd. for C14H9F3N4O6 (386.24) C, 43.54; H, 2.35: N, 14.51. Found: C, 43.66; H, 2.23; N, 14.38%. IR: 3539, 3347 ʋ(O–H); 3270, ʋ(N–H); 1637, ʋ(C=N); 1536, ʋas(NO2); 1268, ʋs(NO2); 1126, ʋ(CF3). 1H NMR (300 MHz, DMSO): δ = 11.45 (s, 1H, NH), 9.49 (s, 2H, OH-b, OH-c), 8.55 (s, 2H, H-3′, H-5′), 8.34 (s, 1H, H-a), 6.42 (d, J = 2.1 Hz, 2H, H-2, H-6), 6.32 (dd, J = 2.1 Hz, 1H, H-4). 13C NMR (75 MHz, DMSO): δ = 158.63, 149.63, 137.33, 135.66, 135.20, 127.19 (q, J = 17.8 Hz), 122.67 (q, J = 271.5 Hz), 116.60 (q, J = 35.4 Hz), 105.48, 105.01; negative LC-MS m/z: 385.0 [M − H]− calc. for C14H8F3N4O6−, 385.04, found 385.0.
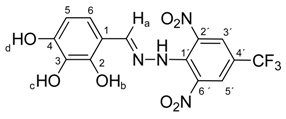
N-(2,3,4-Dihydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazine (5f). Yield 85%; red solid, M.p. 232–233 °C, Anal. Calcd. for C14H9F3N4O7 (402.24) C, 41.80; H, 2.26: N, 13.93. Found: C, 41.70; H, 2.18; N, 14.05%. IR: 3532, 3481, 3359 ʋ(O–H); 3272, ʋ(N–H); 1633, ʋ(C=N); 1530, ʋas(NO2); 1269, ʋs(NO2); 1128, ʋ(CF3). 1H NMR (300 MHz, DMSO): δ = 11.48 (s, 1H, NH), 9.70 (s, 1H, OH-b), 9.03 (s, 1H, OH-d), 8.67 (s, 1H, OH-c), 8.54 (s, 1H, H-a), 8.50 (s, 2H, H-3′, H-5′), 6.85 (d, J = 8.6 Hz, 1H, H-6), 6.37 (d, J = 8.6 Hz, 1H, H-5). 13C NMR (151 MHz, DMSO): δ = 160.34, 149.53, 137.47, 136.16, 129.54, 127.64 (q, J = 3.3 Hz), 125.11, 123.17 (q, J = 271.5 Hz), 116.37 (q, J = 35.1 Hz), 116.32; negative LC-MS m/z: 401.0 [M − H]− calc. for C14H8F3N4O7−, 401.035, found 401.0.
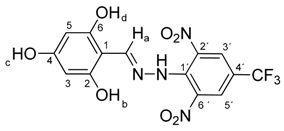
N-(2,4,6-Dihydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazine (5g). Yield 84%; red solid, M.p. 238–240 °C, Anal. Calcd. for C14H9F3N4O7 (402.24) C, 41.80; H, 2.26: N, 13.93. Found: C, 41.96; H, 2.30; N, 13.80%. IR: 3323 ʋ(O–H); 3262, ʋ(N–H); 1633, ʋ(C=N); 1532, ʋas(NO2); 1263, ʋs(NO2); 1126, ʋ(CF3). 1H NMR (300 MHz, DMSO): δ = 11.44 (s, 1H, NH), 10.02 (s, 1H, OH-c), 9.83 (s, 2H, OH-b, OH-d), 8.89 (s, 1H, H-a), 8.54 (s, 2H, H-3′, H-5′), 5.86 (s, 2H, H-3, H-5). 13C NMR (75 MHz, DMSO): δ = 162.57, 159.57, 150.75 (q, J = 14.5 Hz), 137.13, 135.08, 127.72 (q, J = 18.9 Hz), 122.68 (q, J = 271.5 Hz), 115.91 (q, J = 35.4 Hz), 98.57, 94.47; negative LC-MS m/z: 401.0 [M − H]− calc. for C14H8F3N4O7−, 401.035, found 401.0.
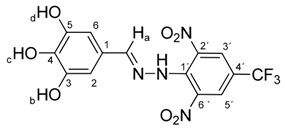
N-(3,4,5-Trihydroxybenzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazine (5h). Yield 71%; red solid, M.p. 279–280 °C, Anal. Calcd. for C14H9F3N4O7 (402.24) C, 41.80; H, 2.26: N, 13.93. Found: C, 41.62; H, 2.15; N, 13.80%. IR: 3428 ʋ(O–H); 3266, ʋ(N–H); 1634, ʋ(C=N); 1537, ʋas(NO2); 1265, ʋs(NO2); 1125, ʋ(CF3). 1H NMR (300 MHz, DMSO): δ = 11.37 (s, 1H, NH), 9.15 (s, 2H, OH-b, OH-d), 8.78 (s, 1H, OH-c), 8.53 (s, 2H, H-3′, H-5′), 8.26 (s, 1H, H-a), 6.53 (s, 2H, H-2, H-6). 13C NMR (75 MHz, DMSO): δ = 150.27, 146.09, 137.01, 136.57, 135.66, 127.23 (q, J = 3.5 Hz), 123.76, 122.73 (q, J = 271.5 Hz), 115.82 (q, J = 35.4 Hz), 106.85; negative LC-MS m/z: 401.0 [M − H]− calc. for C14H8F3N4O7−, 401.035, found 401.0.
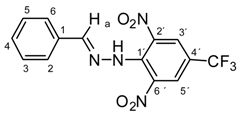
N-(Benzylidene)-N′-[2,6-dinitro-4-(trifluoromethyl)]phenylhydrazine (5i). Yield 70%; red solid, M.p. 231–232 °C, Anal. Calcd. for C14H9F3N4O4 (354.24) C, 47.47; H, 2.56: N, 15.82. Found: C, 47.65; H, 2.48; N, 15.71%. IR: 3265, ʋ(N–H); 1631, ʋ(C=N); 1538, ʋas(NO2); 1267, ʋs(NO2); 1121, ʋ(CF3). 1H NMR (600 MHz, DMSO): δ = 11.57 (s, 1H, NH), 8.57 (s, 2H, H-3′, H-5′), 8.53 (s, 1H, H-a), 7.55 (dd, J = 7.7, 1.4 Hz, 2H, H-2, H-6), 7.50 – 7.42 (m, 3H, H-3, H-4, H-5). 13C NMR (151 MHz, DMSO): δ = 149.02, 137.84, 136.05, 134.15, 130.94, 129.44, 127.64 (q, J = 3.4 Hz), 127.58, 123.10 (q, J = 271.6 Hz), 117.29 (q, J = 35.3 Hz); negative LC-MS m/z: 353.0 [M − H]− calc. for C14H9F3N4O4−, 353.05, found 353.0.
3.3. Free Radical Scavenging Activity Assay
The free radical scavenging activity of the prepared hydroxybenzylidene hydrazines was carried out according to our previous work [25]. Various amounts of tested compounds were added into a methanol solution of DPPH or GOR and the final DPPH or GOR concentration was kept constant (c = 10−4 mol·dm−3) or into a water solution of ABTS. The free radical scavenging activity was evaluated using the values SC50, i.e., the concentration of the studied compound, which causes a 50% decrease in absorbance at 517 nm (for DPPH), 862 nm (for GOR), or 734 nm (for ABTS) as compared to the control sample. Methanol or water was used as a blank. All samples were measured in triplicate. Standard square deviations were in a range from 0.91 to 0.99.
3.4. Photosynthetic Electron Transport (PET) Study
PET was monitored in spinach chloroplasts prepared according to our previous work [4,27]. PET through PSII was monitored by the Hill reaction with DCPIP as an artificial electron acceptor. DCPIP photoreduction was determined spectrophotometrically. The chlorophyll (Chl) concentration in these experiments was 30 mg/dm3. The inhibitory activities of the studied compounds were expressed by IC50 values, i.e., molar concentrations of the compounds causing a 50% decrease of absorbance at 600 nm compared to the control sample. Each sample was measured in triplicate and standard square deviations were in the 0.88–0.94 range.
3.5. Molecular Calculations
The prepared hydroxybenzylidene hydrazines, their anions and radicals were studied using the quantum chemical method PM6 [28], which is part of the program MOPAC2012 [29]. Optimal structures of compounds were calculated (keyword PRECISE). The effect of solvents on the above mentioned compounds were studied by COSMO-method [30], which is also part of MOPAC2012 [31]. Ionization potentials and enthalpy of formations used for the calculation of PDE, BDE, PA, and ETE according to our previous work [25].
Supplementary Materials
The following are available online: Table S1: Proton dissociation energy of prepared N-hydroxybenzylidene hydrazines in methanol; Table S2: Dissociation energy of hydrogen and electron of prepared N-hydroxybenzylidene hydrazines in methanol; Table S3: Proton affinity of prepared N-hydroxybenzylidene hydrazines in methanol; Table S4: Electron transfer enthalpy of prepared N-hydroxybenzylidene hydrazines in methanol; Table S5: Proton dissociation energy of prepared N-hydroxybenzylidene hydrazines in water; Table S6: Dissociation energy of hydrogen and electron of prepared N-hydroxybenzylidene hydrazines in water; Table S7: Proton affinity of prepared N-hydroxybenzylidene hydrazines in water; Table S8: Electron transfer enthalpy of prepared N-hydroxybenzylidene hydrazines in water.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF): (P23609, P21437, and F34), the Slovak Grant Agency VEGA (1/0196/14) and by the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement number PCIG11-GA-2012-322300. We want to thank our colleagues from the Department of Chemistry, Faculty of Natural Sciences, Matej Bell University for measuring IR spectra and from the Research Institute of Molecular Pathology, Vienna, Austria, for their support.
Author Contributions
F.S., F.G. and P.K. performed experiments. F.S., F.G., J.K., J.F., D.L. and J.G. analyzed results and wrote the manuscript. All authors contributed to the paper and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Backes, G.L.; Neumann, D.M.; Jursic, B.S. Synthesis and antifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides. Bioorg. Med. Chem. 2014, 22, 4629–4636. [Google Scholar] [CrossRef] [PubMed]
- Dabideen, D.R.; Cheng, K.F.; Aljabari, B.; Miller, E.J.; Pavlov, V.A.; Al-Abed, Y. Phenolic hydrazones are potent inhibitors of macrophage migration inhibitory factor proinflammatory activity and survival improving agents in sepsis. J. Med. Chem. 2007, 50, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Gadilohar, B.; Shankarling, G. Choline based ionic liquids and their applications in organic transformation. J. Mol. Liq. 2007, 227, 234–261. [Google Scholar] [CrossRef]
- Sersen, F.; Gregan, F.; Pesko, M.; Dvoranova, D.; Kralova, K.; Matkovicova, Z.; Gregan, J.; Donovalova, J. Synthesis and herbicidal activity of new hydrazide and hydrazonoyl derivatives. Molecules 2015, 20, 14139–14154. [Google Scholar] [CrossRef] [PubMed]
- Rollas, S.; Kucukguzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Narang, R.; Narasimhan, B.; Sharma, S. A review on biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.X.; Tang, J.J.; Luo, H.; Jin, X.L.; Dai, F.; Yang, J.; Qian, Y.P.; Li, X.Z.; Zhou, B. Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg. Med. Chem. Lett. 2010, 20, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Torok, B.; Sood, A.; Bag, S.; Tulsan, R.; Ghosh, S.; Borkin, D.; Kennedy, A.R.; Melanson, M.; Madden, R.; Zhou, W.; et al. Diaryl hydrazones as multifunctional inhibitors of amyloid self-assembly. Biochemistry 2013, 52, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.N.; Alafeefy, A.M.; Bakht, M.A.; Masand, V.H.; Aldalbahi, A.; Chen, N.; Fan, C.; Ben Bacha, A. Synthesis, antiphospholipase A(2), antiprotease, antibacterial evaluation and molecular docking analysis of certain novel hydrazones. Molecules 2016, 21, 1664. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Farooq, M.; Khattab, S.N.; Abutaha, N.; Wadaan, M.A.; Ghabbour, H.A.; Fun, H.K. Synthesis, characterization, and anti-cancer activity of some new N′-(2-Oxoindolin-3-ylidene)-2-propylpentane hydrazide-hydrazones derivatives. Molecules 2015, 20, 14638–14655. [Google Scholar] [CrossRef] [PubMed]
- Casanova, B.B.; Muniz, M.N.; de Oliveira, T.; de Oliveira, L.F.; Machado, M.M.; Fuentefria, A.M.; Gosmann, G.; Gnoatto, S.C. Synthesis and biological evaluation of hydrazone derivatives as antifungal agents. Molecules 2015, 20, 9229–9241. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Magana, Y.; Garcia-Ramos, J.C.; Navarro-Olivarria, M.; Flores-Alamo, M.; Manzanera-Estrada, M.; Ortiz-Frade, L.; Galindo-Murillo, R.; Ruiz-Azuara, L.; Melendrez-Luevano, R.M.; Cabrera-Vivas, B.M. Potential amoebicidal activity of hydrazone derivatives: synthesis, characterization, electrochemical behavior, theoretical study and evaluation of the biological activity. Molecules 2015, 20, 9929–9948. [Google Scholar] [CrossRef] [PubMed]
- La Regina, G.; Sarkar, T.; Bai, R.; Edler, M.C.; Saletti, R.; Coluccia, A.; Piscitelli, F.; Minelli, L.; Gatti, V.; Mazzoccoli, C.; et al. New arylthioindoles and related bioisosteres at the sulfur bridging group. 4. Synthesis, tubulin polymerization, cell growth inhibition, and molecular modeling studies. J. Med. Chem. 2009, 52, 7512–7527. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, R.; Cascio, M.G.; La Regina, G.; Piscitelli, F.; Lavecchia, A.; Brizzi, A.; Pasquini, S.; Botta, M.; Novellino, E.; Di Marzo, V.; et al. Synthesis, cannabinoid receptor affinity, and molecular modeling studies of substituted 1-aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. J. Med. Chem. 2008, 51, 1560–1576. [Google Scholar] [CrossRef] [PubMed]
- Baratto, L.C.; Porsani, M.V.; Pimentel, I.C.; Pereira Netto, A.B.; Paschke, R.; Oliveira, B.H. Preparation of betulinic acid derivatives by chemical and biotransformation methods and determination of cytotoxicity against selected cancer cell lines. Eur. J. Med. Chem. 2013, 68, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Singh, A.K. DFT calculations on molecular structure, spectral analysis, multiple interactions, reactivity, NLO property and molecular docking study of flavanol-2,4-dinitrophenylhydrazone. J. Mol. Struct. 2017, 1129, 128–141. [Google Scholar] [CrossRef]
- Parodi, S.; de Flora, S.; Cavanna, M.; Pino, A.; Robbiano, L.; Bennicelli, C.; Brambilla, G. DNA-damaging activity in vivo and bacterial mutagenicity of sixteen hydrazine derivatives as related quantitatively to their carcinogenicity. Cancer Res. 1981, 41, 1469–1482. [Google Scholar] [PubMed]
- Heath, R.L. Hydrazine as an electron donor to the water-oxidation site in photosynthesis. Biochim. Biophys. Acta 1971, 245, 160–164. [Google Scholar] [CrossRef]
- Messinger, J.; Renger, G. Generation, oxidation by the oxidized form of the tyrosine of polypeptide D2, and possible electronic configuration of the redox states S0, S-1, and S-2 of the water oxidase in isolated spinach thylakoids. Biochemistry 1993, 32, 9379–9386. [Google Scholar] [CrossRef] [PubMed]
- Forster, V.; Junge, W. On the action of hydroxylamine, hydrazine and their derivatives on the water-oxidizing complex. Photosynth. Res. 1986, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Messinger, J.; Renger, G. The reactivity of hydrazine with photosystem II strongly depends on the redox state of the water oxidizing system. FEBS Lett. 1990, 277, 141–146. [Google Scholar] [CrossRef]
- Crampton, M.R.; Khan, H.A. The stabilities of meisenheimer complexes. J. Chem. Soc. Perkin Trans. 1972, 2, 1173–1177. [Google Scholar] [CrossRef]
- Sakamoto, H.; Goto, H.; Yokoshima, M.; Dobashi, M.; Ishikawa, J.; Doi, K.; Otomo, M. Benzocrown ether hydrazones as extractants for alkali metal ions. Bull. Chem. Soc. Jpn. 1993, 66, 2907–2914. [Google Scholar] [CrossRef]
- Sersen, F.; Mucaji, P.; Spilkova, J.; Valko, V.; Haladova, M.; Eisenreichova, E.; Grancai, D. Antioxidative effectivness of various solvent extracts from leaves of Cynara cardunculus, Philadelphus coronarius, Lilium candidum, Holodiscus discolor and Ligustrum vulgare. Acta Fac. Pharm. Univ. Comen. 2006, 53, 262–267. [Google Scholar]
- Kotora, P.; Sersen, F.; Filo, J.; Loos, D.; Gregan, J.; Gregan, F. The scavenging of DPPH, galvinoxyl and ABTS radicals by imine analogs of resveratrol. Molecules 2016, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Sersen, F.; Lacova, M. Antioxidant activity of some coumarins. Acta Fac. Pharm. Univ. Comen. 2015, 62, 41–45. [Google Scholar]
- Dolezal, M.; Miletin, M.; Kunes, J.; Kralova, K. Substituted amides of pyrazine-2-carboxylic acids: synthesis and biological activity. Molecules 2002, 7, 363–373. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. MOPAC, version 2012; Stewart Computational Chemistry: Colorado Springs, CO, USA, 2012; Available online: http://openmopac.net/MOPAC2012brochure.pdf.
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 5, 799–805. [Google Scholar] [CrossRef]
- MOPAC Manual. Available online: http://OpenMOPAC.net/Manual (accessed on 10 January 2015).
Sample Availability: Samples of the compounds are not available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).