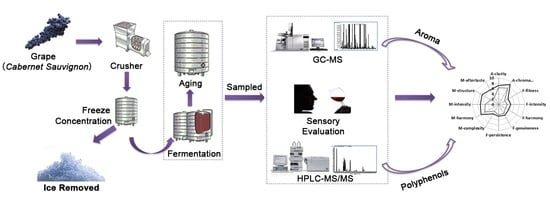
Influence of Freeze Concentration Technique on Aromatic and Phenolic Compounds, Color Attributes, and Sensory Properties of Cabernet Sauvignon Wine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Index
2.2. Volatile Compounds
2.3. Phenolic Compounds
2.3.1. Non-Anthocyanin Phenolic Compounds
2.3.2. Anthocyanins and Color Attributes
2.4. Sensory Evaluation
3. Materials and Methods
3.1. Vinification
3.2. Chemicals and Standards
3.3. Standard Solution
3.4. Volatile Compound Analyses
3.5. Phenolic Compound Analyses
3.6. Color Analysis
3.7. Sensory Evaluation
3.8. Odor Activity Values
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gordillo, B.; Rodríguezpulido, F.J.; Escuderogilete, M.L.; Gonzálezmiret, M.L.; Heredia, F.J. Comprehensive colorimetric study of anthocyanic copigmentation in model solutions. Effects of pH and molar ratio. J. Agric. Food Chem. 2012, 60, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Bíró, I.; Bufa, A.; Kilár, F. Validation of a Method for Analysis of Aroma Compounds in Red Wine using Liquid–Liquid Extraction and GC–MS. Food Anal. Method 2012, 5, 1427–1434. [Google Scholar] [CrossRef]
- Pisarnitskii, A.F. Formation of Wine Aroma: Tones and Imperfections Caused by Minor Components (Review). Appl. Biochem. Microbiol. 2001, 37, 651–659. [Google Scholar] [CrossRef]
- Sánchezpalomo, E.; Garcíacarpintero, E.G.; Alonsovillegas, R.; Gonzálezviñas, M.A. Characterization of aroma compounds of Verdejo white wines from the La Mancha region by odour activity values. Flavour Fragr. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Ugliano, M. Oxygen contribution to wine aroma evolution during bottle aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Capone, D.L.; Wilkinson, K.L.; Jeffery, D.W. Chemical and sensory profiles of rosé wines from Australia. Food Chem. 2016, 196, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Villamor, R.R.; Ross, C.F. Wine matrix compounds affect perception of wine aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Liu, C.H.; Nan, H.J.; Li, Z. Phenolic compound profiles in skins of white wine and table grape cultivars grown in the National Grape Germplasm Resource Nursery of China. S. Afr. J. Enol. Vitic. 2015, 36, 154–164. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, Y.; Liu, D.; Li, Z.; Zhang, X.X.; Li, J.M.; Huang, J.H.; Wang, J.; Pan, Q.H. Evolution of phenolic compounds and sensory in bottled red wines and their co-development. Food Chem. 2015, 172, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Samsuri, S.; Amran, N.A.; Yahya, N.; Jusoh, M. Review on Progressive Freeze Concentration Designs. Chem. Eng. Commun. 2015, 203, 345–363. [Google Scholar] [CrossRef]
- Sánchez, J.; Hernández, E.; Auleda, J.M.; Raventós, M. Freeze concentration of whey in a falling-film based pilot plant: Process and characterization. J. Food Eng. 2011, 103, 147–155. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Sheng, Q.; Chen, J.; Huang, W.; Zhan, J. Effect of Suspension Freeze-concentration Technology on the Quality of Wine. S. Afr. J. Enol. Vitic. 2016, 37, 39–46. [Google Scholar] [CrossRef]
- Cheng, G.; He, Y.N.; Yue, T.X.; Wang, J.; Zhang, Z.W. Effects of climatic conditions and soil properties on Cabernet Sauvignon berry growth and anthocyanin profiles. Molecules 2014, 19, 13683–13703. [Google Scholar] [CrossRef] [PubMed]
- Bonada, M.; Jeffery, D.W.; Petrie, P.R.; Moran, M.A.; Sadras, V.O. Impact of elevated temperature and water deficit on the chemical and sensory profiles of Barossa Shiraz grapes and wines. Aust. J. Grape Wine Res. 2015, 21, 240–253. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.Q.; Wang, Y.H.; Lu, L.; Lan, Y.B.; Reeves, M.J.; Duan, C.Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, X.D. A generalized correlation of solute inclusion in ice formed from aqueous solutions and food liquids on sub-cooled surface. Can. J. Chem. Eng. 2000, 78, 312–319. [Google Scholar] [CrossRef]
- Belén, F.; Sánchez, J.; Hernández, E.; Auleda, J.M.; Raventós, M. One option for the management of wastewater from tofu production: Freeze concentration in a falling-film system. J. Food Eng. 2012, 110, 364–373. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; GB 15037-2006 Wines; Standard Press: Beijing, China, 2006.
- Albertyn, J.; Hohmann, S.; Thevelein, J.M.; Prior, B.A. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 1994, 14, 4135–4144. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Fujii, T.; Nagasawa, N.; Iwamatsu, A.; Bogaki, T.; Tamai, Y.; Hamachi, M. Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl. Environ. Microbiol. 1994, 60, 2786–2792. [Google Scholar] [PubMed]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. Olfactory Impact of Higher Alcohols on Red Wine Fruity Ester Aroma Expression in Model Solution. J. Agric. Food Chem. 2016, 63, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Fleet, G.H.; Heard, G.M. Yeasts: Growth during fermentation. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 27–54. [Google Scholar]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Igor, L.; Sanja, R.; Nikola, G.; Mario, S.; Dordano, P.U. Changes in physico-chemical and volatile aroma compound composition of Gewürztraminer wine as a result of late and ice harvest. Food Chem. 2016, 196, 1048–1057. [Google Scholar]
- Li, H.; Tao, Y.S.; Wang, H.; Zhang, L. Impact odorants of Chardonnay dry white wine from Changli County (China). Eur. Food Res. Technol. 2008, 227, 287–292. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical study of aromatic series in sherry wines subjected to biological aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.S.; Zhang, L.; Hecht, K. Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China). LWT Food Sci. Technol. 2011, 43, 1550–1556. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Volatile compounds of young wines from cabernet sauvignon, cabernet gernischet and chardonnay varieties grown in the loess plateau region of china. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Agric. Food Chem. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Hu, K.; Qin, Y.; Tao, Y.S.; Zhu, X.L.; Peng, C.T.; Ullah, N. Potential of Glycosidase from Non-Saccharomyces Isolates for Enhancement of Wine Aroma. J. Food Sci. 2016, 81, M935–M943. [Google Scholar] [CrossRef] [PubMed]
- Xiaofen, D.; Chad, E.F.; MichaelC, Q. Volatile composition and odour-activity value of thornless ‘Black Diamond’ and ‘Marion’ blackberries. Food Chem. 2010, 119, 1127–1134. [Google Scholar]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache rosé winesroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Feng, T.; Zhuang, H.; Ye, R.; Li, M.; Liu, T. Comparison of Different Extraction Methods in the Analysis of Volatile Compounds in Pomegranate Juice. Food Anal. Methods 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Alañón, M.E.; Schumacher, R.; Castro-Vázquez, L.; Díaz-Maroto, I.J.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Enological potential of chestnut wood for aging Tempranillo wines part I: Volatile compounds and sensorial properties. Food Res. Int. 2013, 51, 325–334. [Google Scholar] [CrossRef]
- Rocha, S.L.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine: Assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Villena, M.A.; Iranzo, J.Ú.; Pérez, A.B. Relationship between Debaryomyces pseudopolymorphus Enzymatic Extracts and Release of Terpenes in Wine. Biotechnol. Prog. 2006, 22, 375–381. [Google Scholar]
- Reboredo-Rodriguez, P.; Gonzalez-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. Effects of sugar concentration processes in grapes and wine aging on aroma compounds of sweet wines—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1053–1073. [Google Scholar] [CrossRef] [PubMed]
- Díaz-maroto, M.C.; Schneider, R.; Baumes, R. Formation Pathways of Ethyl Esters of Branched Short-Chain Fatty Acids during Wine Aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Gammacurta, M.; Marchand, S.; Albertin, W.; Moine, V.; Revel, G.D. Impact of Yeast Strain on Ester Levels and Fruity Aroma Persistence during Aging of Bordeaux Red Wines. J. Agric. Food Chem. 2014, 62, 5378–5389. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; He, F.; Yang, L.; Chen, F.; Reeves, M.J.; Li, J. Comparative study of phenolic compounds in Cabernet Sauvignon wines made in traditional and Ganimede fermenters. Food Chem. 2013, 141, 3984–3992. [Google Scholar] [CrossRef] [PubMed]
- González-Manzano, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Extraction of flavan-3-ols from grape seed and skin into wine using simulated maceration. Anal. Chim. Acta 2004, 513, 283–289. [Google Scholar] [CrossRef]
- Gomez Alonso, S. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar] [CrossRef]
- Bimpilas, A.; Tsimogiannis, D.; Balta-Brouma, K.; Lymperopoulou, T.; Oreopoulou, V. Evolution of phenolic compounds and metal content of wine during alcoholic fermentation and storage. Food Chem. 2015, 178, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, Q.H.; Jin, Z.M.; He, J.J.; Liang, N.N.; Duan, C.Q. Evolution of 49 Phenolic Compounds in Shortly-aged Red Wines Made from Cabernet Gernischt (Vitis vinifera L. cv.). Food Sci. Biotechnol. 2009, 18, 1001–1012. [Google Scholar]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Panprivech, S.; Lerno, L.A.; Brenneman, C.A.; Block, D.E.; Oberholster, A. Investigating the Effect of Cold Soak Duration on Phenolic Extraction during Cabernet Sauvignon Fermentation. Molecules 2015, 20, 7974–7989. [Google Scholar] [CrossRef] [PubMed]
- Heras-Roger, J.; Pomposo-Medina, M.; Díaz-Romero, C.; Darias-Martín, J. Copigmentation, colour and antioxidant activity of single-cultivar red wines. Eur. Food Res. Technol. 2014, 239, 1–7. [Google Scholar] [CrossRef]
- Benítez, P.; Castro, R.; García, B.C. Changes in the Polyphenolic and Volatile Contents of “Fino” Sherry Wine Exposed to Ultraviolet and Visible Radiation during Storage. J. Agric. Food Chem. 2003, 51, 6482–6487. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ribes, A.M.; Leandro, M.C.; Belchior, A.P.; Spranger, M.I. Stilbenes: Quantitative extraction from grape skins, contribution of grape solids to wine and variation during wine maturation. Anal. Chim. Acta 2006, 563, 382–390. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, Q.; Cui, X.; Duan, C. Optimization on anthocyanins extraction from wine grape skins using orthogonal test design. Food Sci. Biotechnol. 2010, 19, 1047–1053. [Google Scholar] [CrossRef]
- Bindon, K.A.; Madani, S.H.; Pendleton, P.; Smith, P.A.; Kennedy, J.A. Factors affecting skin tannin extractability in ripening grapes. J. Agric. Food Chem. 2014, 62, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Marquez, A.; Serratosa, M.P.; Merida, J. Pyranoanthocyanin Derived Pigments in Wine: Structure and Formation during Winemaking. J. Chem. 2013, 1–15. [Google Scholar] [CrossRef]
- Mcrae, J.M.; Dambergs, R.G.; Kassara, S.; Parker, M.; Jeffery, D.W.; Herderich, M.J.; Smith, P.A. Phenolic Compositions of 50 and 30 Year Sequences of Australian Red Wines: The Impact of Wine Age. J. Agric. Food Chem. 2012, 60, 10093–10102. [Google Scholar] [CrossRef] [PubMed]
- Bimpilas, A.; Panagopoulou, M.; Tsimogiannis, D.; Oreopoulou, V. Anthocyanin copigmentation and color of wine: The effect of naturally obtained hydroxycinnamic acids as cofactors. Food Chem. 2016, 197, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors Influencing the Aroma Composition of Chardonnay Wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pan, Q.; Qu, W.; Duan, C. Comparison of volatile profiles of nine litchi (Litchi chinensis Sonn.) cultivars from Southern China. J. Agric. Food. Chem. 2009, 57, 9676–9681. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yang, J.; Ma, L.; Cai, J.; Li, J. Comparison of Polyphenol Profile and Inhibitory Activities Against Oxidation and alpha-Glucosidase in Mulberry (Genus Morus) Cultivars from China. J. Food Sci. 2015, 80, C2440–C2451. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Meudec, E.; Verbaere, A.; Mazerolles, G.; Wirth, J.; Masson, G.; Cheynier, V.; Sommerer, N. A High-Throughput UHPLC-QqQ-MS Method for Polyphenol Profiling in Rose Wines. Molecules 2015, 20, 7890–7891. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the aromatic compounds without * in Table 3, all the non-anthocyanins (except for cis-resveratrol and cis-piecid), malvinidin-3-O-glucosides, peonidin-3-O-glucosides, cyanindin-3-O-glucosides, and delphinidin-3-O-glucosides are available from the authors. |

| Physicochemical Index | Fresh | Concentrated | Mixed | Removed |
|---|---|---|---|---|
| Sugar (g/L) | 228.23 ± 2.96 c | 462.70 ± 9.21 a | 255.71 ± 6.51 b | 62.95 ± 5.43 d |
| Total acid (g/L) | 5.44 ± 0.01 b | 6.31 ± 0.22 a | 5.44 ± 0.05 b | 5.15 ± 0.17 b |
| Volatile acid (g/L) | 0.08 ± 0.01 c | 0.14 ± <0.01 a | 0.10 ± <0.01 b | / |
| pH | 3.78 ± 0.02 a b | 3.74 ± 0.01 b | 3.78 ± 0.03 a b | 3.81 ± 0.01 a |
| Physicochemical Index | Fermentation | One-Year Aging | ||
|---|---|---|---|---|
| Control | Freeze Concentration | Control | Freeze Concentration | |
| Alcohol (%, v/v) | 13.32 ± 0.14 b | 14.41 ± 0.18 a | 13.12 ± 0.16 b | 14.40 ± 0.16 a |
| Reducing sugar (g/L) | 2.00 ± 0.14 a | 1.45 ± 0.07 b | 2.00 ± 0.14 a | 2.35 ± 0.21 a |
| Total acid (g/L) | 6.30 ± 0.28 a b | 6.65 ± 0.35 a | 6.00 ± 0.28 a b | 5.85 ± 0.21 b |
| Volatile acid (g/L) | 0.57 ± 0.02 b | 0.55 ± 0.03 b | 0.69 ± 0.03 a | 0.71 ± 0.04 a |
| pH | 3.82 ± 0.17 a | 4.07 ± 0.18 a | 3.97 ± 0.16 a | 4.14 ± 0.16 a |
| Glycerol (g/L) | 8.60 ± 0.28 b c | 9.60 ± 0.28 a | 8.00 ± 0.28 c | 9.20 ± 0.28 a b |
| Aromatic Compound | Threshold | Odor Description | Ref. | Fermentation | One-Year Aging | ||
|---|---|---|---|---|---|---|---|
| Control | Freeze Concentration | Control | Freeze Concentration | ||||
| Esters | |||||||
| Ethyl acetate | 12,000 | pineapple, varnish, balsamic | [26] | 47,948.68 ± 27.08 c | 56,841.96 ± 1598.83 b | 56,637.07 ± 2876.76 b | 67,394.61 ± 797.76 a |
| Ethyl butanoate | 20 | strawberry, apple, banana | [27] | 310.00 ± 24.08 a b | 401.21 ± 59.90 a | 315.73 ± 36.10 a b | 214.16 ± 11.84 b |
| Isoamyl acetate | 160 | banana, fruity, sweet | [26] | 1168.39 ± 32.31 b | 1618.65 ± 58.36 a | 770.28 ± 8.14 c | 520.52 ± 14.98 d |
| Ethyl hexanoate | 80 | fruity, green apple, banana, brandy, winelike | [26] | 383.38 ± 15.72 b | 544.85 ± 26.57 a | 398.17 ± 5.39 b | 313.98 ± 14.31 c |
| Hexyl acetate | 1500 | fruity, pear | [28] | 4.74 ± 0.35 b | 6.71 ± 0.25 a | 2.92 ± 0.28 c | 1.76 ± 0.21 d |
| Ethyl heptanoate * | 300 | winelike, brandy, fruity | [29] | 0.64 ± 0.06 b | 0.92 ± 0.12 a | 0.60 ± 0.08 b | 1.11 ± 0.02 a |
| Ethyl lactate | 14,000 | fruity, buttery | [15,30] | 2136.21 ± 175.76 b | 2520.77 ± 22.81 a | 2034.38 ± 1.17 b | 2374.45 ± 184.53 a b |
| Isobutyl hexanoate * | 4.17 ± 0.31 b | 5.77 ± 0.14 a | 3.27 ± 0.06 c | 3.16 ± 0.27 c | |||
| Methyl octanoate | 200 | intense citrus | [31] | 2.81 ± 0.13 a | 2.5 ± 0.19 a | 2.50 ± 0.14 a | 1.61 ± 0.06 b |
| Ethyl octanoate | 580 | sweet, floral, fruity, banana, pear, brandy | [26] | 616.91 ± 27.32 a | 696.89 ± 49.33 a | 607.38 ± 40.03 a | 438.71 ± 7.53 b |
| Isoamyl hexanoate | 1000 | Pineapple, cheese | [28] | 5.25 ± 0.21 a b | 5.52 ± 0.11 a | 5.19 ± 0.08 a b | 5.03 ± 0.01 b |
| Propyl octanoate * | 2.78 ± 0.19 a | 2.72 ± 0.21 a | 3.49 ± 0.55 a | 1.44 ± 0.10 b | |||
| Ethyl nonanoate | 1300 | Rose, fruity | [28] | 2.25 ± 0.12 a | 1.76 ± 0.04 a | 2.65 ± 0.97 a | 2.54 ± <0.01 a |
| Ethyl 2-hydroxy-4-methyl pentanate * | 12.59 ± 0.17 c | 10.31 ± 0.40 c | 42.14 ± 1.51 b | 50.19 ± 1.00 a | |||
| Isobutyl octanoate * | 800 | [28] | 5.25 ± 0.52 a b | 5.92 ± 0.33 a | 4.81 ± 0.40 b | 3.08 ± 0.21 c | |
| Isoamyl lactate | 200 | buttery, nut | [30] | 119.12 ± 12.93 d | 157.74 ± 4.28 c | 320.45 ± 7.38 b | 372.32 ± 7.52 a |
| Methyl decanoate * | 1200 | [28] | 3.03 ± 0.28 a b | 2.83 ± 0.18 a b | 3.51 ± 0.45 a | 2.32 ± 0.08 b | |
| Ethyl decanoate | 200 | grape, fruit | [27] | 284.87 ± 10.63 b | 335.91 ± 5.92 a | 298.78 ± 31.9 a b | 235.01 ± 2.79 c |
| Isoamyl octanoate | 1000 | Sweet, light fruity, cheese, cream | [28] | 37.14 ± 0.83 b | 43.37 ± 1.41 a | 33.83 ± 2.73 b | 24.83 ± 1.16 c |
| Ethyl benzoate | 575 | [25] | 0.82 ± 0.01 b | 0.35 ± 0.04 c | 1.29 ± 0.07 a | 0.75 ± 0.01 b | |
| Diethyl succinate | 6000 | overripe melon, lavender, vinous | [26] | 161.95 ± 20.7 c | 275.01 ± 36.86 c | 843.63 ± 54.47 b | 1185.03 ± 158.12 a |
| Ethyl 9-decenoate | 100 | green, fruity, fatty | [32] | 6.19 ± 0.78 a b | 28.78 ± 16.12 a | 4.56 ± 0.19 b | 7.54 ± 1.20 a b |
| Propyl decanoate * | 0.45 ± 0.03 b | 0.62 ± 0.10 a | nd | nd | |||
| Ethyl undecanoate * | 0.62 ± 0.06 a | 0.71 ± 0.06 a | 0.49 ± 0.29 a | 0.30 ± <0.01 a | |||
| Methyl salicylate | 40 | [33] | 3.90 ± 0.15 c | 2.12 ± 0.01 c | 24.46 ± 1.61 a | 12.52 ± 0.68 b | |
| Ethyl phenylacetate | rose, floral | [20] | 1.24 ± 0.06 c | 1.11 ± <0.01 c | 1.81 ± 0.06 b | 3.53 ± 0.07 a | |
| Ethyl salicylate | 11.64 ± <0.01 b | 11.32 ± 0.02 c | 11.86 ± 0.04 a | 11.81 ± 0.04 a | |||
| Phenethyl acetate | 1800 | fruity | [26] | 33.82 ± 0.55 a | 33.61 ± 2.40 a | 24.50 ± 0.35 c | 28.06 ± 0.23 b |
| Ethyl dodecanoate | 500 | fruity, floral | [29] | 58.37 ± 4.40 a b | 68.87 ± 4.29 a | 47.41 ± 7.66 b c | 34.97 ± 2.25 c |
| Isopentyl decanoate * | 33.42 ± 5.48 b | 50.44 ± 0.10 a | 30.2 ± 6.91 b | 23.56 ± 3.73 b | |||
| Ethyl dihydrocinnamate * | 1.6 | sweet, pleasant | [34] | 3.17 ± 0.08 a | nd | 3.33 ± 0.22 a | 2.36 ± 0.05 b |
| Ethyl myristate * | 500 | mild waxy, soapy | [29] | 2.22 ± 0.39 a | 1.09 ± 0.11 b | 1.71 ± 0.42 a b | 1.35 ± 0.13 b |
| Ethyl palmitate * | 1000 | mild waxy | [29] | 1.65 ± 0.35 a | 1.45 ± 0.13 a | 1.32 ± 0.41 a | 1.08 ± 0.01 a |
| Total (mg/L) | 53.37 ± 0.06 c | 63.68 ± 1.75 b | 62.48 ± 3.07 b | 73.27 ± 1.17 a | |||
| Alcohols | |||||||
| 1-Propanol | 306,000 | alcohol, ripe fruit | [26] | 53,963.28 ± 818.91 b | 63,554.43 ± 2075.83 a | 55,066.05 ± 3306.10 b | 54,425.82 ± 856.53 b |
| Isobutanol | 75,000 | alcohol, nail polish | [26] | 46,796.40 ± 388.87 b | 50,229.69 ± 1074.77 a b | 46,078.47 ± 2717.74 b | 51,738.37 ± 1485.45 a |
| 1-Butanol | 150,000 | medicinal, phenolic | [26] | 1564.43 ± 32.26 b | 1511.50 ± 39.64 b | 1731.64 ± 62.71 a | 1740.55 ± 8.08 a |
| Isopentanol | 60,000 | nail polish, alcohol | [26] | 240,695.90 ± 197.47 b | 274,678.10 ± 9597.97 a | 242,018.97 ± 7302.91 b | 291,089.97 ± 8184.10 a |
| 1-Pentanol | 64,000 | almond, balsam | [4] | 106.36 ± 10.08 a | 109.19 ± 5.01 a | 102.24 ± 6.20 a | 109.62 ± 5.33 a |
| 4-Methyl-1-pentanol | 50,000 | almond, toasty | [4] | 63.73 ± 3.10 b | 71.55 ± 1.17 a | 61.89 ± 0.80 b | 75.73 ± 1.75 a |
| 3-Methyl-1-pentanol | 50,000 | vinous, herbaceous, cacao | [4] | 157.40 ± 0.62 a b | 165.77 ± 1.48 a | 151.50 ± 0.28 b | 156.37 ± 6.72 a b |
| 1-Hexanol | 1100 | herbaceous | [26] | 2554.32 ± 32.42 c | 3221.72 ± 100.59 a | 2774.28 ± 48.47 b | 3048.03 ± 52.86 a |
| (E)-3-Hexen-1-ol | 400 | green, floral | [31] | 73.70 ± 2.73 b c | 92.23 ± 3.61 a | 80.61 ± 3.08 c | 85.94 ± 0.79 a b |
| (Z)-3-Hexen-1-ol | 400 | green | [31] | 40.89 ± 3.57 b | 39.80 ± 3.85 b | 71.16 ± 2.25 a | 67.32 ± 0.52 a |
| 3-Octanol * | 0.96 ± 0.04 a b | 0.84 ± <0.01 b | 1.02 ± 0.06 a | 0.99 ± 0.07 a | |||
| (E)-2-Hexen-1-ol | 400 | green grass, herb | [31] | nd | nd | 52.09 ± 1.31 a | 50.46 ± 0.76 a |
| 1-Octen-3-ol | 20 | mushroom | [15] | 4.72 ± 0.14 b | 3.50 ± 0.14 c | 7.69 ± 0.60 a | 7.85 ± 0.35 a |
| 2-Ethyl-1-hexanol | 8000 | mushroom, sweet fruity | [31] | 0.47 ± 0.16 c | 1.01 ± 0.04 b c | 1.14 ± 0.33 b | 6.38 ± 0.17 a |
| 3-Ethyl-4-methyl pentanol * | 94.31 ± 1.03 c | 82.69 ± 2.16 c | 144.34 ± 6.14 b | 284.78 ± 11.70 a | |||
| 2-Nonanol | 58 | coconut | [31] | 1.16 ± 0.04 b | 0.95 ± 0.04 c | 1.75 ± 0.06 a | 1.74 ± 0.08 a |
| 1-Octanol | 800 | jasmine, lemon | [26] | 21.24 ± 0.23 b | 24.14 ± 0.37 a | 25.06 ± 1.07 a | 23.59 ± 0.09 a |
| 1-Nonanol * | 600 | [30] | 45.30 ± 0.65 c | 47.00 ± 1.50 b c | 50.72 ± 2.20 b | 68.54 ± 1.09 a | |
| (Z)-6-Nonen-1-ol | 3.39 ± 0.01 a | 2.57 ± 0.30 b | 3.58 ± 0.27 a | 3.75 ± 0.34 a | |||
| Methionol | 1500 | cooked potato, garlic | [26] | 1366.57 ± 12.59 a b | 918.91 ± 10.76 c | 1277.70 ± 105.32 b | 1503.03 ± 21.60 a |
| 1-Decanol | 500 | floral, fruity, waxy, fatty | [29] | 4.45 ± 0.04 a b | 3.54 ± 0.18 c | 4.87 ± 0.40 a | 3.94 ± 0.02 b c |
| Benzyl alcohol | 900,000 | roasted, toasted | [26] | 664.65 ± 7.16 b | 625.89 ± 26.81 b | 2227.26 ± 232.54 a | 2635.85 ± 194.02 a |
| 2-Phenylethanol | 14,000 | rose, honey | [15] | 39,049.57 ± 149.55 a | 34,466.48 ± 625.96 c | 39,525.08 ± 428.78 a | 36,755.50 ± 1107.36 b |
| 1-Dodecanol | 1000 | unpleasant odor, fruity | [32] | 28.82 ± 0.71 a b | 17.93 ± 1.21 c | 35.00 ± 5.52 a | 24.77 ± 0.27 b c |
| Total (mg/L) | 387.30 ± 8.66 b | 429.87 ± 9.39 a | 391.49 ± 7.59 b | 443.91 ± 10.20 a | |||
| Acids | |||||||
| Acetic acid | 200,000 | sour, pungent, vinegar | [27] | 835.24 ± 59.97 b | 586.37 ± 40.95 c | 1053.23 ± 140.12 a | 1101.65 ± 126.00 a |
| Propanoic acid | 8100 | pungent, rancid, soy | [15] | 1288.69 ± 10.23 a | 467.10 ± 60.68 b | 1233.23 ± 167.68 a | 166.30 ± 0.82 c |
| Isobutyric acid | 2300 | pungent, rancid, soy | [15] | 629.42 ± 43.37 a | 682.41 ± 10.66 a | 647.43 ± 4.71 a | 626.32 ± 38.08 a |
| Isovaleric acid | 3000 | rancid | [15] | 648.75 ± 73.56 c | 807.17 ± 3.61 b | 540.34 ± 47.71 c | 1049.51 ± 18.05 a |
| Hexanoic acid | 420 | rancid, cheese | [27] | 1758.46 ± 59.97 a b | 1658.15 ± 49.67 b | 2053.87 ± 143.66 a | 1302.42 ± 160.19 c |
| Octanoic acid | 500 | rancid, cheese, fatty | [27] | 1118.91 ± 12.80 b | 799.53 ± 9.29 c | 1556.45 ± 112.66 a | 789.14 ± 36.13 c |
| Decanoic acid | 1000 | rancid, fatty | [27] | 576.75 ± 56.04 b | 471.12 ± 58.04 c | 850.88 ± 38.43 a | 472.15 ± 4.68 c |
| Total (mg/L) | 6.86 ± 0.16 b | 5.47 ± 0.21 c | 7.94 ± 0.58 a | 5.51 ± 0.37 c | |||
| Terpenes | |||||||
| β-Myrcene | 15 | fruity, grape, wine-like | [33,35] | 0.87 ± 0.01 a | 0.83 ± <0.01 a | 0.86 ± 0.07 a | 0.84 ± 0.03 a |
| Linalool | 15 | citrus, floral, sweet, grape-like | [26] | 1.16 ± 0.03 c | 1.27 ± 0.03 c | 1.98 ± 0.12 b | 2.56 ± 0.06 a |
| α-Terpineol | 250 | lilac, floral, sweet | [15] | 0.71 ± 0.03 b | 1.26 ± 0.39 a b | 1.11 ± 0.07 a b | 1.41 ± 0.02 a |
| β-Citronellol | 100 | rose | [26] | 6.47 ± 0.08 a b | 6.59 ± 0.12 a | 6.17 ± 0.12 b | 5.54 ± 0.16 c |
| Geraniol | 30 | roses, geranium | [4] | 124.61 ± 10.75 a b | 147.46 ± 6.45 a | 107.33 ± 14.81 b c | 82.84 ± 7.74 c |
| Nerol | 15 | orange flowers, rose | [27] | 36.89 ± 1.10 a | 38.28 ± 2.95 a | 35.75 ± 0.01 a | 37.21 ± 0.35 a |
| Total (µg/L) | 170.70 ± 11.69 a b | 195.86 ± 8.86 a | 153.18 ± 15.07 b c | 130.39 ± 7.15 c | |||
| C13-norisoprenoids | |||||||
| β-Damascenone | 0.14 | floral, stewed apple | [15] | 0.75 ± 0.04 a | 0.77 ± 0.02 a | 0.64 ± 0.13 a b | 0.49 ± 0.05 b |
| Geranylacetone | 60 | flora | [31] | 0.71 ± 0.05 b | 1.08 ± 0.08 a | 0.77 ± 0.11 b | 0.66 ± 0.01 b |
| Total | 1.46 ± 0.01 b | 1.85 ± 0.10 a | 1.40 ± 0.24 b | 1.16 ± 0.04 b | |||
| Aldehydes | |||||||
| Nonanal | 1 | herbaceous | [36] | 0.29 ± 0.13 a | 0.48 ± 0.04 a | 0.45 ± 0.57 a | 0.90 ± 0.09 a |
| Furfural | 14,100 | toasty | [31] | tr | tr | 20.66 ± 1.17 b | 137.98 ± 1.85 a |
| Decanal | 1000 | grassy, orange skin | [31] | 1.91 ± 0.02 b | 1.87 ± 0.04 b | 2.13 ± 0.29 b | 3.74 ± 0.06 a |
| Benzealdehyde | 2000 | toasty, almond | [31] | 19.84 ± 0.25 c | 17.85 ± 0.04 d | 20.58 ± 0.09 b | 21.24 ± 0.31 a |
| Dodecanal | 3.51 ± 0.01 a | tr | 3.52 ± 0.06 a | tr | |||
| Total (µg/L) | 25.54 ± 0.42 c | 20.2 ± 0.05 c | 47.32 ± 0.34 b | 163.85 ± 2.32 a | |||
| Volatile phenols | |||||||
| Phenol | 25,000 | phenolic | [37] | 39.66 ± 0.97 b | 19.83 ± 1.52 d | 51.37 ± 3.42 a | 27.59 ± 0.37 c |
| Others | |||||||
| Styrene * | 7.33 ± 0.26 b | 4.66 ± 0.18 c | 6.88 ± 0.21 c | 4.97 ± 0.06 a | |||
| Acetoin | 150,000 | buttery, fatty | [26] | 5845.39 ± 95.08 b | 798.1 ± 4.56 c | 1338.62 ± 413.99 c | 11,827.10 ± 956.3 a |
| Naphthalene * | 1.10 ± 0.03 b | 1.34 ± 0.01 a | 1.15 ± 0.01 b | 1.33 ± 0.04 a | |||
| Non-Anthocyanin | Fermentation | One-Year Aging | ||
|---|---|---|---|---|
| Control | Freeze Concentration | Control | Freeze Concentration | |
| Flavan-3-ols | ||||
| Catechin | 46.91 ± 5.39 b | 64.70 ± 6.28 a | 40.44 ± 4.16 b | 38.44 ± 2.90 b |
| Epicatechin | 12.79 ± 1.02 b | 18.21 ± 3.09 a | 12.86 ± 1.10 b | 12.89 ± 1.56 b |
| Procyanidin B2 | 16.27 ± 0.22 a | 15.10 ± 0.58 a | 14.62 ± 2.20 a | 17.66 ± 1.93 a |
| Procyanidin B1 | 13.41 ± 1.05 a | 9.43 ± 0.97 b | 14.22 ± 1.69 a | 14.00 ± 1.47 a |
| (−)-Gallocatechin | 2.53 ± 0.11 a | 2.67 ± 0.33 a | 2.20 ± 0.24 a | 2.19 ± 0.26 a |
| (−)-Epigallocatechin | 2.36 ± 0.44 a | 1.74 ± 0.34 a | 2.05 ± 0.34 a | 1.59 ± 0.16 a |
| Epicatechin gallate | 0.24 ± 0.01 c | 1.28 ± 0.22 a | 0.60 ± 0.10 b | 0.22 ± 0.01 c |
| Total | 94.51 ± 8.02 b | 113.13 ± 9.56 a | 87.00 ± 9.83 b | 87.00 ± 8.26 b |
| Flavonols | ||||
| Quercetin-3-O-glucuronide | 13.13 ± 1.5 a | 11.06 ± 1.42 a | 9.97 ± 1.25 a | 10.71 ± 1.13 a |
| Quercetin-3-O-glucoside | 5.26 ± 0.30 a | 5.94 ± 0.41 a | 2.67 ± 0.58 b | 2.69 ± 0.30 b |
| Quercetin | 7.03 ± 0.79 b | 2.97 ± 0.19 c | 9.54 ± 0.71 a | 10.04 ± 1.23 a |
| Myricetin | 2.99 ± 0.43 b | 2.32 ± 0.17 b | 4.42 ± 0.45 a | 5.55 ± 0.60 a |
| Isorhamnetin-3-O-glucoside | 1.55 ± 0.13 a b | 1.71 ± 0.2 a | 1.24 ± 0.28 a b | 1.04 ± 0.16 b |
| Quercetin-3-O-galactoside | 1.76 ± 0.17 a | 1.68 ± 0.22 a | 1.19 ± 0.09 b | 0.90 ± 0.13 b |
| Quercetin-3-O-rutinoside | nd | 1.37 ± 0.23 a | 0.12 ± 0.01 b | 0.09 ± 0.01 b |
| Kaempferol-3-O-glucoside | 0.64 ± 0.09 b | 1.16 ± 0.09 a | 0.20 ± 0.01 c | 0.19 ± 0.01 c |
| Quercetin-3-O-rhamnetin | nd | 0.32 ± 0.04 a | 0.31 ± 0.04 a | 0.36 ± 0.05 a |
| Taxifolin | 0.49 ± 0.06 a | 0.31 ± 0.05 b | 0.37 ± 0.04 a b | 0.45 ± 0.03 a |
| Isorhamnetin | 0.21 ± 0.03 c | 0.07 ± 0.01 d | 0.34 ± 0.02 b | 0.42 ± 0.02 a |
| Kaempferol | tr | tr | 0.19 ± 0.02 b | 0.38 ± 0.06 a |
| Total | 33.05 ± 3.00 a | 28.93 ± 2.47 a | 30.55 ± 3.20 a | 32.83 ± 3.69 a |
| Phenolic acids | ||||
| Gallic acid | 16.57 ± 0.95 a | 13.98 ± 1.96 b | 4.91 ± 0.47 c | 4.63 ± 0.50 c |
| Syringic acid | 2.12 ± 0.21 b | 2.75 ± 0.31 a | 1.62 ± 0.32 b | 2.15 ± 0.04 b |
| Gentisic acid | 0.96 ± 0.08 b | 1.29 ± 0.05 a | 0.09 ± 0.02 c | 0.13 ± 0.01 c |
| Caffeic acid | 3.22 ± 0.20 c | 0.96 ± 0.09 d | 8.90 ± 0.15 a | 6.77 ± 0.35 b |
| Vanillic acid | 0.48 ± 0.05 a b | 0.53 ± 0.06 a | 0.30 ± 0.05 c | 0.36 ± 0.06 b c |
| Ferulic acid | 0.17 ± 0.01 c | 0.18 ± 0.02 c | 0.76 ± 0.06 b | 0.98 ± 0.04 a |
| p-Hydroxybenzoic acid | 0.03 ± <0.01 c | 0.05 ± <0.01 b | 0.05 ± <0.01 b | 0.09 ± 0.01 a |
| p-Coumaric acid | 0.25 ± 0.02 b | 0.03 ± <0.01 c | 1.41 ± 0.21 a | 1.62 ± 0.17 a |
| Total | 23.79 ± 1.51 a | 19.76 ± 1.76 b | 18.03 ± 0.96 b | 16.72 ± 1.05 b |
| Stilbenes | ||||
| cis-Piceid | 1.62 ± 0.26 a | 1.38 ± 0.09 a | 0.76 ± 0.16 b | 0.95 ± 0.06 b |
| trans-Piceid | 1.08 ± 0.03 a | 0.93 ± 0.15 a | 0.16 ± 0.01 c | 0.49 ± 0.06 b |
| cis-Resveratrol | 1.77 ± 0.32 a | 1.83 ± 0.13 a | 1.39 ± 0.09 b | 0.57 ± 0.03 c |
| trans-Resveratrol | 0.95 ± 0.14 a b | 1.14 ± 0.06 a | 0.71 ± 0.13 b | 0.42 ± 0.04 c |
| Picentannol | 0.34 ± 0.03 a | 0.17 ± 0.02 b | 0.10 ± 0.01 c | 0.14 ± 0.02 b c |
| Total | 5.76 ± 0.79 a | 5.46 ± 0.07 a | 3.12 ± 0.38 b | 2.57 ± 0.06 b |
| Anthocyanin | Quant ** | Fermentation | One-Year Aging | ||
|---|---|---|---|---|---|
| Control | Freeze Concentration | Control | Freeze Concentration | ||
| Non-acylated anthocyanins | |||||
| Delphinidin-3,5-O-diglucoside | I | 1.15 ± 0.09 c | 1.78 ± 0.11 a | 1.18 ± 0.13 c | 1.43 ± 0.11 b |
| Peonidin-3,5-O-diglucoside | II | 0.09 ± 0.01 b | 0.15 ± 0.01 a | 0.09 ± 0.01 b | 0.10 ± 0.01 b |
| Delphinidin-3-O-glucoside | III | 15.50 ± 1.20 b c | 27.34 ± 1.58 a | 10.52 ± 0.82 d | 12.81 ± 1.18 cd |
| Malvinidin-3,5-O-diglucoside * | IV | 32.44 ± 2.16 a | tr | tr | 18.78 ± 3.70 a |
| Cyanidin-3-O-glucoside | V | 2.29 ± 0.17 b | 3.24 ± 0.27 a | 1.42 ± 0.16 c | 2.39 ± 0.21 b |
| Petunidin-3-O-glucoside | IV | 40.27 ± 4.16 b | 48.07 ± 2.58 a | 19.01 ± 1.35 c | 22.40 ± 1.50 c |
| Peonidin-3-O-glucoside | II | 9.06 ± 0.86 a | 8.36 ± 0.50 a b | 5.05 ± 0.32 c | 7.57 ± 0.48 b |
| Malvinidin-3-O-glucoside | IV | 95.00 ± 1.72 a | 93.78 ± 4.84 a | 62.02 ± 2.99 c | 74.32 ± 4.37 b |
| Total | 163.40 ± 4.77 b | 182.73 ± 6.17 a | 99.29 ± 3.07 d | 121.04 ± 3.90 c | |
| Acylated anthocyanins | |||||
| Delphinidin-3-O-(6-O-acetyl)-glucoside | III | 6.45 ± 2.96 b | 10.21 ± 0.64 a | 4.41 ± 0.35 b | 4.92 ± 0.30 b |
| Cyanidin-3-O-(6-O-acetyl)-glucoside | V | 3.45 ± 0.29 a | 3.39 ± 0.30 a | 1.33 ± 0.10 b | 1.62 ± 0.17 b |
| Petunidin-3-O-(6-O-acetyl)-glucoside | IV | 16.07 ± 1.29 a | 16.25 ± 1.67 a | 6.60 ± 0.43 b | 7.93 ± 0.45 b |
| Peonidin-3-O-(6-O-acetyl)-glucoside | II | 7.16 ± 0.67 a | 5.80 ± 0.34 b | 2.32 ± 0.26 c | 3.02 ± 0.29 c |
| Malvinidin-3-O-(6-O-acetyl)-glucoside | IV | 40.87 ± 3.01 a | 43.51 ± 2.63 a | 16.82 ± 1.08 b | 21.11 ± 1.37 b |
| cis-Delphinidin-3-O-(6-O-coumaryl)-glucoside | III | 1.13 ± 0.08 a | 0.67 ± 0.05 b | 0.21 ± 0.01 c | 0.21 ± 0.02 c |
| trans-Delphinidin-3-O-(6-O-coumaryl)-glucoside * | III | 78.40 ± 8.18 a | 36.16 ± 1.96 b | 12.63 ± 0.77 c | 16.32 ± 1.42 c |
| cis-Petunidin-3-O-(6-O-coumaryl)-glucoside * | IV | 71.12 ± 8.36 a | 82.83 ± 9.20 a | 16.11 ± 2.10 b | 12.49 ± 2.86 b |
| trans-Petunidin-3-O-(6-O-coumaryl)-glucoside | IV | 1.51 ± 0.09 a | 0.93 ± 0.07 b | 0.25 ± <0.01 a | 0.27 ± 0.02 b |
| Peonidin-3-O-(6-O-caffeoyl)-glucoside * | II | 38.93 ± 2.98 a | 20.79 ± 1.18 b | 5.78 ± 0.53 c | 10.13 ± 0.67 c |
| Malvidin-3-O-(6-O-caffeoyl)-glucoside * | IV | 107.63 ± 12.23 a | 90.46 ± 5.50 a | 21.42 ± 4.13 b | 31.28 ± 1.90 b |
| cis-Peonidin-3-O-(6-O-coumaryl)-glucoside * | II | 85.06 ± 7.23 a | 46.15 ± 3.96 b | 19.38 ± 1.20 c | 19.97 ± 1.56 c |
| trans-Peonidin-3-O-(6-O-coumaryl)-glucoside | II | 1.61 ± 0.19 a | 0.92 ± 0.07 b | 0.31 ± 0.02 c | 0.35 ± 0.02 c |
| Malvinidin-3-O-(6-O-coumaryl)-glucoside | IV | 5.91 ± 0.32 a | 5.02 ± 0.45 b | 1.49 ± 0.12 c | 1.59 ± 0.15 c |
| Total | 84.54 ± 2.38 a | 86.97 ± 4.17 a | 33.82 ± 0.65 c | 41.10 ± 1.29 b | |
| Pyranoanthocyanins | |||||
| Petunidin-3-O-glucoside-pyruvate | IV | 0.17 ± 0.02 a | 0.15 ± 0.02 a | 0.14 ± 0.01 a | 0.17 ± 0.01 a |
| Malvinidin-3-O-glucoside-pyruvic acid | IV | 0.95 ± 0.07 a | 0.84 ± 0.05 a b | 0.69 ± 0.05 b c | 0.86 ± 0.06 a |
| Malvinidin-3-O-glucoside-acetaldehyde | IV | 1.07 ± 0.11 b | 6.78 ± 0.39 a | 0.80 ± 0.07 b c | 0.30 ± 0.02 c |
| Peonidin-3-O-(6-O-acetyl)-glucoside-4-vinylphenol * | II | 61.39 ± 6.9 a | 47.36 ± 3.17 b | 31.28 ± 4.40 c | 41.12 ± 2.28 b c |
| Malvinidin-3-O-(6-O-acetyl)-glucoside-pyruvic acid | IV | 0.42 ± 0.03 a | 0.34 ± 0.02 b | 0.23 ± 0.02 c | 0.31 ± 0.03 b |
| Malvinidin-3-O-(6-O-acetyl)-glucoside-acetaldehyde | IV | 0.26 ± 0.01 b | 2.37 ± 0.31 a | 0.21 ± 0.02 b | 0.07 ± <0.01 b |
| Malvinidin-3-O-(6-O-coumaryl)-glucoside-acetaldehyde * | VI | 50.07 ± 3.68 b | 309.55 ± 20.28 a | 30.80 ± 1.58 b c | 12.17 ± 1.09 c |
| Petunidin-3-O-glucoside-4-vinylphenol * | IV | 93.07 ± 7.62 a | 16.50 ± 1.60 b | 45.31 ± 3.10 a | 28.18 ± 1.80 a b |
| Malvinidin-3-O-glucoside-vinylguaiacol * | IV | 45.77 ± 5.04 a | 7.15 ± 0.37 c | 20.22 ± 2.17 b | 17.71 ± 0.97 b |
| Malvinidin-3-O-glucoside-4-vinylphenol | IV | 0.78 ± 0.09 a | 0.16 ± 0.01 c | 0.45 ± 0.02 b | 0.29 ± 0.02 c |
| Peonidin-3-O-(6-O-acetyl)-glucoside-4-viniylphenol * | II | 36.28 ± 3.49 a | 5.80 ± 1.08 b | 8.83 ± 1.05 b | 6.50 ± 0.59 b |
| Malvinidin-3-O-(6-O-acetyl)-glucoside-4-vinylphenol * | IV | 157.31 ± 16.91 a | 36.10 ± 4.01 b | 56.89 ± 4.67 b | 39.14 ± 4.24 b |
| Malvinidin-3-O-(6-O-coumaryl)-glucoside-vinylphenol * | IV | 26.36 ± 4.64 a | 3.55 ± 0.32 b | 8.55 ± 0.93 b | 5.22 ± 0.41 b |
| Total | 4.11 ± 0.25 b | 11.05 ± 0.58 a | 2.71 ± 0.05 c | 2.15 ± 0.07 c | |
| Polymeric anthocyanin | |||||
| (epi)catechin-ethyl-Malvidin-3-O-glucoside isomer 1 * | IV | 4.94 ± 0.35 b | 8.71 ± 1.12 a | 1.18 ± 0.03 c | 0.84 ± 0.10 c |
| (epi)catechin-ethyl-Malvidin-3-O-glucoside isomer 2 * | IV | 12.18 ± 1.56 a | 12.70 ± 1.13 a | 2.17 ± 0.13 b | 1.52 ± 0.14 b |
| (epi)catechin-ethyl-Malvidin-3-O-glucoside isomer 3 * | IV | 12.42 ± 0.12 a | 9.79 ± 0.54 b | 3.61 ± 0.14 c | 3.09 ± 0.17 c |
| (epi)catechin-ethyl-Malvidin-3-O-glucoside isomer 4 * | IV | 14.40 ± 0.67 b | 20.32 ± 0.84 a | 1.98 ± 0.13 c | 1.31 ± 0.13 c |
| Total | 0.04 ± <0.01 a | 0.05 ± <0.01 b | 0.01 ± <0.01 c | 0.01 ± <0.01 c | |
| Color Attribute | Fermentation | One-Year Aging | ||
|---|---|---|---|---|
| Control | Freeze Concentration | Control | Freeze Concentration | |
| L* | 65.52 ± 3.70 a | 52.37 ± 3.05 b | 61.61 ± 3.93 a | 62.64 ± 3.83 a |
| a* | 37.3 ± 2.09 b c | 49.37 ± 3.10 a | 32.69 ± 1.72 c | 36.98 ± 2.19 b c |
| b* | 5.97 ± 0.47 c | 3.61 ± 0.20 d | 19.32 ± 1.44 a | 13.40 ± 0.97 b |
| C* | 37.78 ± 0.22 b | 49.51 ± 0.11 a | 37.97 ± 0.04 b | 39.34 ± 0.19 b |
| H | 9.10 ± 0.67 c | 4.18 ± 0.16 d | 30.59 ± 0.88 a | 19.91 ± 0.15 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-Y.; Xing, K.; Zhang, X.-X.; Wang, H.; Wang, Y.; Wang, F.; Li, J.-M. Influence of Freeze Concentration Technique on Aromatic and Phenolic Compounds, Color Attributes, and Sensory Properties of Cabernet Sauvignon Wine. Molecules 2017, 22, 899. https://doi.org/10.3390/molecules22060899
Wu Y-Y, Xing K, Zhang X-X, Wang H, Wang Y, Wang F, Li J-M. Influence of Freeze Concentration Technique on Aromatic and Phenolic Compounds, Color Attributes, and Sensory Properties of Cabernet Sauvignon Wine. Molecules. 2017; 22(6):899. https://doi.org/10.3390/molecules22060899
Chicago/Turabian StyleWu, Yan-Yan, Kai Xing, Xiao-Xu Zhang, Hui Wang, Yong Wang, Fang Wang, and Jing-Ming Li. 2017. "Influence of Freeze Concentration Technique on Aromatic and Phenolic Compounds, Color Attributes, and Sensory Properties of Cabernet Sauvignon Wine" Molecules 22, no. 6: 899. https://doi.org/10.3390/molecules22060899
APA StyleWu, Y. -Y., Xing, K., Zhang, X. -X., Wang, H., Wang, Y., Wang, F., & Li, J. -M. (2017). Influence of Freeze Concentration Technique on Aromatic and Phenolic Compounds, Color Attributes, and Sensory Properties of Cabernet Sauvignon Wine. Molecules, 22(6), 899. https://doi.org/10.3390/molecules22060899