Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae)
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the EO
2.2. Acetic-Acid-Writhing-Induced Nociception
2.3. Formalin-Induced Nociception
2.3.1. Involvement of Muscarinic Receptors
2.3.2. Involvement of ATP-Sensitive K+ Channels
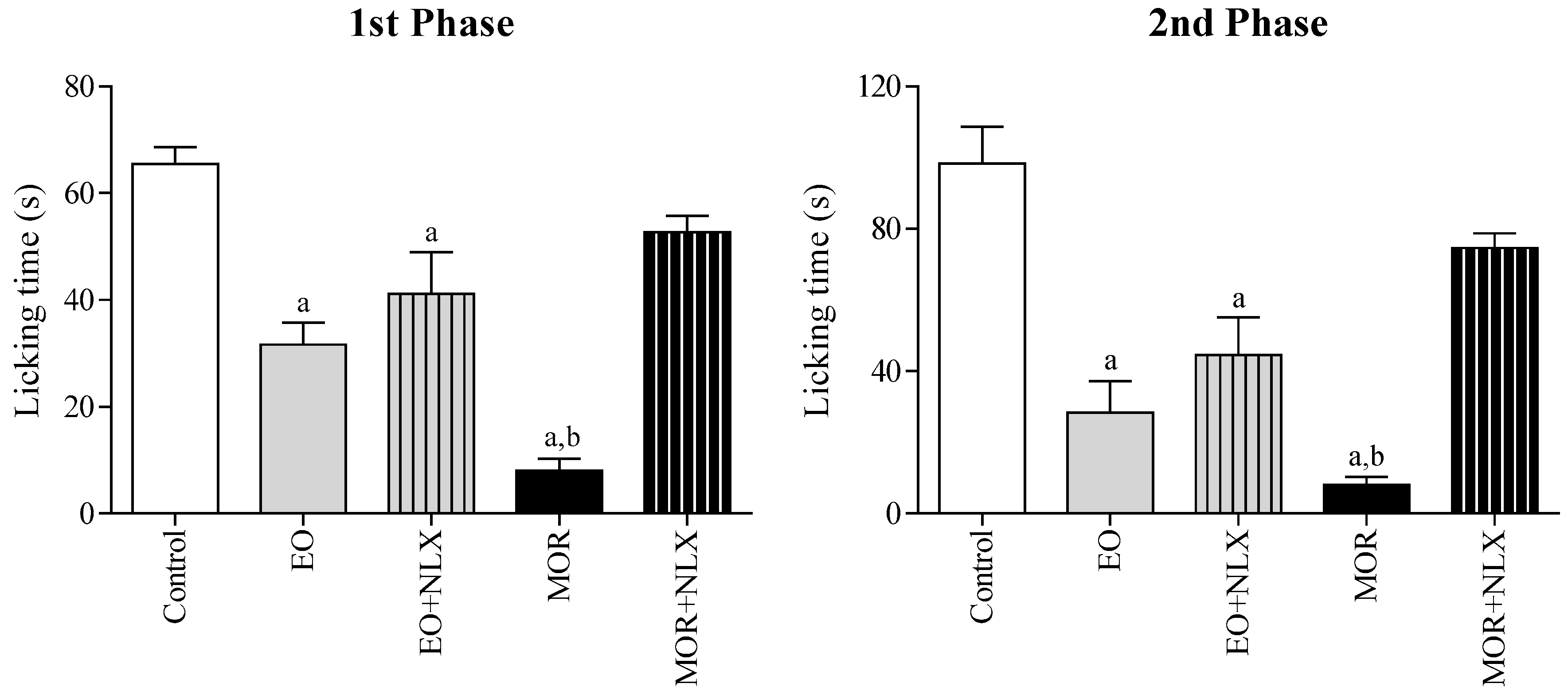
2.3.3. Involvement of Opioid Receptors
2.4. Hot Plate Test
2.5. Leukocyte Migration to the Peritoneal Cavity
2.6. Rota-Rod Test
2.7. Docking Study
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Extraction
4.3. Chemical Analysis of the Essential Oil (the EO)
4.4. Animals
4.5. Drug Treatments
4.6. Acetic-Acid-Writhing-Induced Nociception
4.7. Formalin-Induced Nociception
4.8. Hot Plate Test
4.9. Leukocyte Migration to the Peritoneal Cavity
4.10. Rota-Rod Test
4.11. Docking Study
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 2014, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, O.; Torrance, N.; Smith, B.H. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013, 111, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Croft, P.; Blyth, F.M.; Windt, D. Chronic Pain Epidemiology from Aetiology to Public Health; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.S.; Speciali, J.G. Epidemiology of chronic pain in the office of a pain specialist neurologist. Arq. Neuropsiquiatr. 2015, 73, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.; Alegre, C.; Blake, D.; Alén, J.C.; Caporali, R.; Casser, H.R.; Correa-Illanes, G.; Fernandes, P.; Galilea, E.; Jany, R. Current Considerations for the Treatment of Severe Chronic Pain. Pain Pract. 2012, 12, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Droney, J.M.; Gretton, S.K.; Sato, H.; Ross, J.R.; Branford, R.; Welsh, K.I.; Cookson, W.; Riley, J. Analgesia and central side-effects: Two separate dimensions of morphine response. Br. J. Clin. Pharmacol. 2013, 75, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Vella-Brincat, J.; Macleod, A.D. Adverse effects of opioids on the central nervous systems of palliative care patients. J. Pain Palliat. Care Pharmacother. 2007, 21, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.T.; Duong, V.; Ho, S.; Ngo, K.C.; Greer, C.L.; Weeks, D.L. Side effects of commonly prescribed analgesic medications. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Lenardão, E.J.; Savegnago, L.; Jacob, R.G.; Victoria, F.N.; Martinez, D.M. Antinociceptive Effect of Essential Oils and Their Constituents: An Update Review. J. Braz. Chem. Soc. 2016, 27, 435–474. [Google Scholar] [CrossRef]
- Anaya-Eugenio, G.D.; Rivero-Cruz, I.; Bye, R.; Linares, E.; Mata, R. Antinociceptive activity of the essential oil from Artemisia ludoviciana. J. Ethnopharmacol. 2016, 179, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Suna, L.; Zong, S.B.; Li, J.C.; Lv, Y.Z.; Liu, L.N.; Wang, Z.Z.; Zhou, J.; Cao, L.; Kou, J.P.; Xiao, W. The essential oil from the twigs of Cinnamomum. cassia Presl alleviates pain and inflammation in mice. J. Ethnopharmacol. 2016, 194, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jung, S.M.; Yoo, S.A.; Kim, W.U.; Cho, C.S.; Park, B.J.; Wood, J.M.; Yoon, C.H. Antinociceptive and anti-inflammatory effects of essential oil extracted from Chamaecyparis. obtusa in mice. Int. Immunopharmacol. 2015, 29, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.G.S.; Souza, A.V.V.; Oliveira, A.P.; Santos, U.S.; Souza, M.D.; Bispo, L.P.; Turatti, I.C.C.; Lopes, N.P. Chemical Composition of Essential Oils from Croton conduplicatus (Euphorbiaceae) in Two Different Seasons. J. Essent. Oil Bear. Plant 2015, 17, 1137–1145. [Google Scholar] [CrossRef]
- Cartaxo, S.L.; Souza, M.M.A.; Albuquerque, U.P. Medicinal plants with bioprospecting potential used in semi-arid northestern Brazil. J. Ethnopharmacol. 2010, 131, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.G.S.; Souza, A.V.V.; Oliveira, A.P.; Santos, U.S.; Souza, M.D.; Bispo, L.P.; Turatti, I.C.C.; Lopes, N.P. Chemical composition of the essential oils from the stem barks of Cronton. conduplicatus (Euphorbiaceae) native to the Caatinga biome. Afr. J. Pharm. Pharmacol. 2015, 9, 98–101. [Google Scholar]
- Katsuyama, S.; Mizoguchi, H.; Kuwahata, H.; Komatsu, T.; Nagaoka, K.; Nakamura, H.; Bagetta, G.; Sakurada, T.; Sakurada, S. Involvement of peripheral cannabinoid and opioid receptors in β-caryophyllene-induced antinociception. Eur. J. Pain 2013, 17, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona. squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Gawade, S.P. Acetic acid induced painful endogenous infliction in writhing test on mice. J. Pharmacol. Pharmacother. 2012, 3, 348. [Google Scholar] [CrossRef] [PubMed]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, D.; Yang, S.; Wang, Y.; Xu, L.; Wu, J.; Ren, J.; Yao, W.; Fan, L.; Zhang, C.; et al. Role of ATP-sensitive potassium channels in modulating nociception in rat model of bone cancer pain. Brain Res. 2014, 1554, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.A.; Castro, M.S.A.; Francischi, J.N.; Perez, A.C.; Duarte, I.D.G. Participation of ATP sensitive K+ channels in the peripheral antinociceptive effect of fentanyl in rats. Braz. J. Med. Biol. Res. 2005, 38, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, D.F.; Garcia-Guzman, M. Muscarinic pain pharmacology: Realizing the promise of novel analgesics by overcoming old challenges. Handb. Exp. Pharmacol. 2012, 208, 191–221. [Google Scholar]
- Wess, J.; Duttaroy, A.; Gomeza, J.; Zhang, W.; Yamada, M.; Felder, C.C.; Bernardini, N.; Reeh, P.W. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with muscarinic receptor knockout mice. Life Sci. 2003, 72, 2047–2054. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Chen, S.R.; Han, H.D.; Sood, A.K.; Lopez-Berestein, G.; Pan, H.L. Role of M2, M3, and M4 Muscarinic Receptor Subtypes in the Spinal Cholinergic Control of Nociception Revealed Using siRNA in Rats. J. Neurochem. 2009, 111, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
- Dool, H.V.; Kratz, P.D.J.A. Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Koster, R.; Anderson, M.; Beer, E.J. Acetic acid for analgesic screening. Fed. Proceedings 1959, 18, 412–418. [Google Scholar]
- Almeida, J.R.G.S.; Souza, G.R.; Silva, J.C.; Lima-Saraiva, S.R.G.; Oliveira-Junior, R.G.; Quintans, J.S.S.; Barreto, R.S.S.; Bonjardim, L.R.; Cavalcanti, S.C.H.; Quintans-Junior, L.J. Borneol, a Bicyclic Monoterpene Alcohol, Reduces Nociceptive Behavior and Inflammatory Response in Mice. Sci. World. J. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.S.; Guimarães, A.G.; Santana, M.T.; Siqueira, R.S.; Passos, L.O.; Machado, S.M.F.; Ribeiro, A.S.; Sobral, M.; Almeida, J.R.G.S.; Quintans-Junior, L.J. Phytochemical screening, antinociceptive and anti-inflammatory effects of the essential oil of Myrcia. pubiflora in mice. Braz. J. Pharmacogn. 2012, 22, 181–188. [Google Scholar] [CrossRef]
- Silva, J.C.; Araújo, C.S.; Lima-Saraiva, S.R.G.; Oliveira-Junior, R.G.; Diniz, T.C.; Wanderley, C.W.S.; Palheta-Junior, R.C.; Mendes, R.L.; Guimarães, A.G.; Quintans-Junior, L.J.; et al. Antinociceptive and anti-inflammatory activities of the ethanolic extract of Annona vepretorum Mart. (Annonaceae) in rodents. BMC Complement. Altern. Med. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Kruse, A.C.; Asada, H.; Yurugi-Kobayashi, T.; Shiroishi, M.; Zhang, C.; Weis, W.I.; Okada, T.; Kobilka, B.K.; Haga, T.; et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 2012, 482, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.C.; Hu, J.; Pan, A.C.; Arlow, D.H.; Rosenbaum, D.M.; Rosemond, E.; Green, H.F.; Liu, T.; Chae, P.S.; Dror, R.O.; et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 2012, 482, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.M.; Sun, B.; Feng, D.; Nawaratne, V.; Leach, K.; Felder, C.C.; Bures, M.G.; Evans, D.A.; Weis, W.I.; Bachhawat, P.; et al. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature 2016, 531, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. Moldock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the the EO (essential oil from stem-bark of C. conduplicatus) are available from the authors. |








| Peak | RT (min) | RI | Compound | % GC-MS |
|---|---|---|---|---|
| 1 | 8.44 | 914 | α-Thujene | 0.04 |
| 2 | 8.91 | 926 | α-Pinene | 5.57 |
| 3 | 9.45 | 941 | Camphene | 0.97 |
| 4 | 10.56 | 969 | β-Pinene | 4.88 |
| 5 | 12.76 | 1026 | 1,8-Cineole | 2.56 |
| 6 | 14.54 | 1072 | cis-Linalool oxide | 0.31 |
| 7 | 15.02 | 1085 | NI | 0.22 |
| 8 | 15.13 | 1088 | trans-Linalool oxide | 1.20 |
| 9 | 15.74 | 1104 | Linalool | 0.74 |
| 10 | 15.81 | 1106 | NI | 0.72 |
| 11 | 16.04 | 1112 | exo-Fenchol | 0.56 |
| 12 | 16.97 | 1137 | cis-Pinocarveol | 0.51 |
| 13 | 17.09 | 1140 | Camphor | 8.25 |
| 14 | 17.28 | 1145 | Camphene hydrate | 0.36 |
| 15 | 17.84 | 1161 | Pinocarvone | 0.16 |
| 16 | 17.99 | 1165 | Borneol | 1.29 |
| 17 | 18.48 | 1178 | Terpinen-4-ol | 1.05 |
| 18 | 19.08 | 1194 | α-Terpineol | 0.62 |
| 19 | 19.11 | 1195 | Myrtenal | 0.89 |
| 20 | 20.55 | 1236 | Thymol, methyl ether | 0.34 |
| 21 | 24.79 | 1362 | Cyclosativene | 0.33 |
| 22 | 25.17 | 1373 | α-Copaene | 0.97 |
| 23 | 25.91 | 1396 | α-Gurjunene | 0.39 |
| 24 | 26.55 | 1417 | (E)-Caryophyllene | 13.72 |
| 25 | 26.87 | 1427 | NI | 0.43 |
| 26 | 27.62 | 1451 | α-Humulene | 6.05 |
| 27 | 27.84 | 1458 | Allo-aromadendrene | 0.23 |
| 28 | 28.07 | 1465 | 9-epi-(E)-Caryophyllene | 0.19 |
| 29 | 28.36 | 1475 | Germacrene D | 4.01 |
| 30 | 28.64 | 1484 | NI | 0.31 |
| 31 | 29.10 | 1498 | α-Muurolene | 3.66 |
| 32 | 29.38 | 1508 | β-Bisabolene | 0.26 |
| 33 | 29.50 | 1512 | γ-Cadinene | 0.17 |
| 34 | 29.79 | 1522 | δ-Cadinene | 0.65 |
| 35 | 30.23 | 1537 | NI | 0.39 |
| 36 | 30.61 | 1550 | Elemol | 0.81 |
| 37 | 31.52 | 1581 | Caryophyllene oxide | 13.15 |
| 38 | 31.99 | 1597 | Guaiol | 2.51 |
| 39 | 32.10 | 1601 | Rosifoliol | 0.83 |
| 40 | 32.28 | 1607 | Humulene epoxide II | 4.19 |
| 41 | 32.44 | 1613 | Eudesmol | 1.94 |
| 42 | 32.78 | 1625 | Muurolol | 3.17 |
| 43 | 33.01 | 1633 | NI | 3.04 |
| 44 | 33.11 | 1637 | NI | 1.83 |
| 45 | 33.48 | 1650 | Agarospirol | 3.01 |
| 46 | 33.74 | 1660 | NI | 1.19 |
| 47 | 33.90 | 1665 | NI | 0.97 |
| 48 | 40.62 | 1926 | NI | 0.35 |
| Total identified | 90.55 | |||
| Compounds | 3UON | 4DAJ | 5DSG |
|---|---|---|---|
| Camphor | −70.79 | −68.33 | −75.02 |
| Caryophyllene oxide | −110.91 | −114.83 | −119.26 |
| (E)-Caryophyllene | −99.35 | −101.76 | −105.77 |
| QNB | −49.49 | −146.61 | −146.30 |
| Tiotropium | –162.89 | −167.60 | −177.81 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Oliveira Júnior, R.G.; Ferraz, C.A.A.; Silva, J.C.; De Oliveira, A.P.; Diniz, T.C.; E Silva, M.G.; Quintans Júnior, L.J.; De Souza, A.V.V.; Dos Santos, U.S.; Turatti, I.C.C.; et al. Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae). Molecules 2017, 22, 900. https://doi.org/10.3390/molecules22060900
De Oliveira Júnior RG, Ferraz CAA, Silva JC, De Oliveira AP, Diniz TC, E Silva MG, Quintans Júnior LJ, De Souza AVV, Dos Santos US, Turatti ICC, et al. Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae). Molecules. 2017; 22(6):900. https://doi.org/10.3390/molecules22060900
Chicago/Turabian StyleDe Oliveira Júnior, Raimundo Gonçalves, Christiane Adrielly Alves Ferraz, Juliane Cabral Silva, Ana Paula De Oliveira, Tâmara Coimbra Diniz, Mariana Gama E Silva, Lucindo José Quintans Júnior, Ana Valéria Vieira De Souza, Uiliane Soares Dos Santos, Izabel Cristina Casanova Turatti, and et al. 2017. "Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae)" Molecules 22, no. 6: 900. https://doi.org/10.3390/molecules22060900
APA StyleDe Oliveira Júnior, R. G., Ferraz, C. A. A., Silva, J. C., De Oliveira, A. P., Diniz, T. C., E Silva, M. G., Quintans Júnior, L. J., De Souza, A. V. V., Dos Santos, U. S., Turatti, I. C. C., Lopes, N. P., Lorenzo, V. P., & Almeida, J. R. G. d. S. (2017). Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae). Molecules, 22(6), 900. https://doi.org/10.3390/molecules22060900







