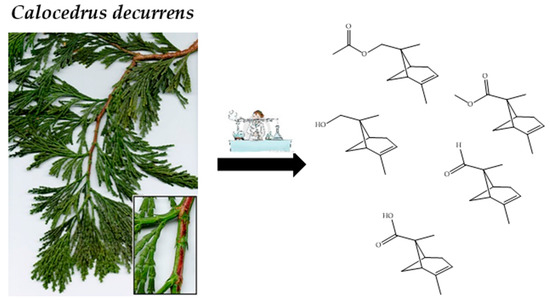
New Pinane Derivatives Found in Essential Oils of Calocedrus decurrens
Abstract
:1. Introduction
2. Results
2.1. Analysis of C. decurrens Leaf, Old Branches and Young Branches Essential Oils by GC(RI), GC-MS and 13C-NMR
2.2. Structure Elucidation of Unidentified Compounds
2.2.1. Identification of Pin-2-en-8-ol (A)
2.2.2. Identification of Pin-2-en-8-yl Acetate (B)
2.2.3. Identification of Pin-2-en-8-al (C)
2.2.4. Identification of Methyl Pin-2-en-8-oate (D)
3. Discussion
4. Materials and Methods
4.1. Plant Material, Isolation of Essential Oils
4.2. Fractionation of the Young Branches Essential Oil
4.3. Preparation of pin-2-en-8-al (C)
4.4. Gas Chromatography
4.5. Gas Chromatography-Mass Spectrometry in Electron Impact Mode
4.6. Preparative Capillary-Gas Chromatography
4.7. Gas Chromatography-High Resolution Mass Spectrometry
4.8. Nuclear Magnetic Resonance
4.9. Identification and Quantification of Individual Components
4.10. Spectral Data
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nam, A.M.; Casanova, J.; Tomi, F.; Bighelli, A. Composition and Chemical Variability of the Corsican Pinus halepensis Cone Oil. Nat. Prod. Commun. 2014, 9, 1361–1364. [Google Scholar] [PubMed]
- Nam, A.M.; Tomi, F.; Gibernau, M.; Casanova, J.; Bighelli, A. Composition and Chemical Variability of the Needle Oil from Pinus. halepensis growing in Corsica. Chem. Biodivers. 2016, 13, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Garcia, A.; Gibernau, M.; Bighelli, A.; Tomi, F. Chemical compositions of essential oils of five introduced conifers in Corsica. Nat. Prod. Res. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Veluthoor, S.; Kelsey, R.G.; González-Hernández, M.P.; Panella, N.; Dolan, M.; Karchesy, J. Composition of the heartwood essential oil of incense cedar (Calocedrus decurrens Torr.). Holzforschung 2011, 65, 333–336. [Google Scholar] [CrossRef]
- Alexandrian, D.; Le Voyer, B. Choix des essences et techniques de reboissement, 2ème rencontres de Forêt Méditerranéenne. Valorisation des patromoines forestiers méditerranéens. Forêt Méditerr. 1984, 6, 89–122. [Google Scholar]
- Nakatsuka, T.; Hirose, Y. The essential oil from the wood of incense cedar, on the phenolic substances. J. Jpn. For. Soc. 1954, 36, 280–283. [Google Scholar]
- Von Rudloff, E. The leaf oil terpene composition of incense cedar and coast redwood. Can. J. Chem. 1981, 59, 285–287. [Google Scholar] [CrossRef]
- Adams, R.P.; Nguyen, S.; Hsieh, C.F.; Kaiyun, G. The Leaf Essential Oils of the Genus Calocedrus. J. Essent. Oil Res. 2006, 18, 654–658. [Google Scholar] [CrossRef]
- Tomi, F.; Bradesi, P.; Bighelli, A.; Casanova, J. Computer-aided identification of individual components of essential oils using carbon-13 NMR spectroscopy. J. Magn. Reson. Anal. 1995, 1, 25–34. [Google Scholar]
- Coxon, J.M.; Hydes, G.J.; Steel, P.J. Carbon-13 Nuclear Magnetic Resonance Spectra of Pinane Monoterpenoids. J. Chem. Soc. Perkin Trans. II 1984, 1351–1355. [Google Scholar] [CrossRef]
- Bessiere, Y.; Grison, C.; Boussac, G. Transpositions thermiques de dérivés d’α-pinène. Tetrahedron 1978, 34, 1957–1963. [Google Scholar] [CrossRef]
- De Pascual Teresa, J.; Urones, J.G.; Fernandez, A. Monoterpene derivatives from the essential oil of Aristolochia Longa. Phytochemistry 1983, 22, 2753–2754. [Google Scholar] [CrossRef]
- Bradesi, P.; Tomi, F.; Terriaga, I.; Casanova, J. Identification of dihydrocarveol stereoisomers and their acetates using carbon-13 NMR spectroscopy. Spectrosc. Lett. 1994, 27, 921–933. [Google Scholar] [CrossRef]
- Groupe Arboretum. Les arboretum méditerranéens. Plan de situation. Forêt Méditerr. 1993, 14, 74–99. [Google Scholar]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau., A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour. Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
- König, W.A.; Hochmuth, D.H.; Joulain, D. Terpenoids and Related Constituents of Essential Oils; Library of MassFinder 2.1; Institute of Organic Chemistry: Hamburg, Germany, 2001. [Google Scholar]
- McLafferty, W.; Stauffer, D.B. Wiley Registry of Mass Spectral Data, 6th ed.; Mass Spectrometry Library Search System Bench-Top/PBM, Version 3.10d; Palisade: Newfield, UK, 1994. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation Company: Carol Stream, IL, USA, 2007. [Google Scholar]
Sample Availability: Samples of the compounds are not anymore available from the authors. |


| No. | Compounds | RIa | RIp | RRF Calculated | L EO | OB EO | YB EO | Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 1-(2-Methylene cyclopropyl) cyclopentene | 870 | 1054 | 0.789 | 0.9 | 0.1 | 0.3 | RI, MS |
| 2 | Tricyclene | 920 | 1015 | 0.765 | 0.1 | 0.3 | 0.2 | RI, MS |
| 3 | α-Thujene | 922 | 1015 | 0.765 | 0.1 | 0.1 | 0.1 | RI, MS |
| 4 | α-Pinene | 932 | 1015 | 0.765 | 11.2 | 56.6 | 22.3 | RI, MS, 13C-NMR |
| 5 | α-Fenchene | 941 | 1047 | 0.765 | 0.7 | 0.2 | 0.2 | RI, MS, 13C-NMR |
| 6 | Camphene | 943 | 1063 | 0.765 | 0.2 | 0.5 | 0.2 | RI, MS, 13C-NMR |
| 7 | Sabinene | 964 | 1122 | 0.765 | 0.1 | 0.3 | 0.2 | RI, MS |
| 8 | β-Pinene | 970 | 1110 | 0.765 | 0.4 | 1.2 | 0.6 | RI, MS, 13C-NMR |
| 9 | 2-Pentylfuran | 976 | - | 0.912 | - | tr | - | RI, MS |
| 10 | Myrcene | 981 | 1159 | 0.765 | 13.4 | 8.4 | 9.6 | RI, MS, 13C-NMR |
| 11 | 1,3,8-p-Menthatriene | 995 | - | 0.779 | - | tr | - | RI, MS |
| 12 | α-Phellandrene | 996 | 1163 | 0.765 | 0.1 | 0.1 | 0.1 | RI, MS |
| 13 | p-Methylanisole | 998 | 1452 | 0.838 | - | tr | - | RI, MS |
| 14 | Δ-3-Carene | 1005 | 1146 | 0.765 | 31.3 | 5.2 | 11.1 | RI, MS, 13C-NMR |
| 15 | α-Terpinene | 1009 | 1178 | 0.765 | 0.2 | 0.1 | 0.2 | RI, MS |
| 16 | p-Cymene | 1011 | 1269 | 0.698 | 0.3 | 0.2 | 0.2 | RI, MS, ¹³C-NMR |
| 17 | Sylvestrene | 1017 | 1199 | 0.765 | 0.5 | 0.1 | 0.2 | RI, MS |
| 18 | Limonene * | 1021 | 1200 | 0.765 | 6.4 | 5.1 | 5.5 | RI, MS, 13C-NMR |
| 19 | β-Phellandrene * | 1021 | 1209 | 0.765 | 1.7 | 1.2 | 1.4 | RI, MS, 13C-NMR |
| 20 | γ-Terpinene | 1048 | 1243 | 0.765 | 0.3 | 0.2 | 0.2 | RI, MS |
| 21 | Methyl phenylethyl ether | 1058 | - | 0.821 | - | 0.1 | 0.1 | RI, MS |
| 22 | Fenchone | 1068 | 1401 | 0.887 | tr | 0.1 | 0.1 | RI, MS |
| 23 | p-Cymenene | 1072 | 1434 | 0.709 | 0.3 | 0.1 | 0.2 | RI, MS, ¹³C-NMR |
| 24 | Terpinolene | 1078 | 1280 | 0.765 | 6.9 | 1.5 | 3.2 | RI, MS, 13C-NMR |
| 25 | Linalool | 1083 | 1543 | 0.869 | 0.2 | tr | 0.2 | RI, MS |
| 26 | Perillene | 1086 | 1419 | 0.907 | - | tr | tr | RI, MS |
| 27 | Pin-2-en-8-al (C) | 1102 | 1460 | 0.907 | 1.6 | 0.8 | 2.0 | RI, MS, 2D-NMR |
| 28 | Camphre | 1120 | 1513 | 0.887 | 0.1 | 0.2 | 0.3 | RI, MS |
| 29 | trans-Pinocarveol | 1123 | 1549 | 0.876 | - | 0.1 | - | RI, MS |
| 30 | trans-p-Menth-2-en-1-ol | 1125 | - | 0.869 | - | - | tr | RI, MS |
| 31 | trans-Verbenol | 1128 | 1680 | 0.876 | 0.1 | tr | 0.1 | RI, MS |
| 32 | Camphene hydrate | 1132 | 1591 | 0.869 | 0.1 | 0.3 | 0.4 | RI, MS |
| 33 | trans-Pinocamphone | 1133 | 1507 | 0.887 | - | - | tr | RI, MS |
| 34 | Myrtenyl methyl ether | 1145 | 1380 | 0.868 | tr | 0.2 | 0.2 | RI, MS, ¹³C-NMR |
| 35 | Borneol | 1150 | 1698 | 0.869 | 0.1 | - | 0.3 | RI, MS |
| 36 | Isopinocamphone | 1153 | 1547 | 0.887 | - | - | tr | RI, MS |
| 37 | p-Methylacetophenone | 1155 | 1776 | 0.839 | 0.1 | - | - | RI, MS |
| 38 | p-Cymen-8-ol | 1159 | 1803 | 0.809 | 0.2 | 0.2 | 0.3 | RI, MS |
| 39 | Terpinen-4-ol | 1161 | 1598 | 0.869 | 1.0 | 0.6 | 1.1 | RI, MS, 13C-NMR |
| 40 | (E)-Dec-4-enal | 1170 | 1537 | 0.869 | 0.7 | 0.2 | 0.6 | RI, MS, 13C-NMR |
| 41 | α-Terpineol | 1172 | 1692 | 0.869 | 0.2 | 0.3 | 0.3 | RI, MS |
| 42 | Myrtenol | 1178 | 1787 | 0.887 | - | - | tr | RI, MS |
| 43 | Verbenone | 1181 | 1708 | 0.907 | 0.3 | 0.4 | 0.6 | RI, MS, 13C-NMR |
| 44 | α-Campholenol | 1186 | 1782 | 0.887 | 0.1 | 0.3 | 0.5 | RI, MS, 13C-NMR |
| 45 | Pin-2-en-8-ol (A) | 1189 | 1800 | 0.887 | 4.2 | 4.5 | 10.3 | RI, MS, 2D-NMR |
| 46 | Methyl pin-2-en-8-oate (D) | 1207 | 1543 | 1.006 | 3.0 | 1.8 | 3.6 | RI, MS, 2D-NMR |
| 47 | Thymyl methyl ether | 1213 | 1589 | 0.798 | - | - | tr | RI, MS |
| 48 | Carvone | 1214 | 1733 | 0.907 | 0.1 | 0.3 | 0.5 | RI, MS, 13C-NMR |
| 49 | Methyl campholenate | 1222 | 1576 | 0.985 | 0.2 | 0.2 | 0.5 | RI, MS, 13C-NMR |
| 50 | Carvacryl methyl ether | 1225 | 1601 | 0.798 | - | - | tr | RI, MS |
| 51 | Piperitone | 1226 | 1730 | 0.887 | 0.3 | 0.3 | 0.5 | RI, MS, 13C-NMR |
| 52 | (Z)-Dec-4-en-1-ol | 1240 | 1789 | 0.852 | - | - | tr | RI, MS |
| 53 | Bornyl acetate | 1269 | 1576 | 0.958 | 0.4 | 0.5 | 0.7 | RI, MS, 13C-NMR |
| 54 | Methyl myrtenate | 1273 | 1685 | 1.006 | 2.3 | 1.6 | 3.5 | RI, MS, 13C-NMR |
| 55 | (E,E)-Deca-2,4-dienal | 1288 | - | 0.887 | 0.2 | - | 0.2 | RI, MS |
| 56 | Myrtenyl acetate | 1305 | 1680 | 0.976 | - | - | tr | RI, MS |
| 57 | Pin-2-en-8-yl Acetate (B) | 1310 | 1683 | 0.976 | 0.6 | 0.6 | 1.4 | RI, MS, 2D-NMR |
| 58 | α-Terpinyl acetate | 1332 | 1690 | 0.958 | 2.4 | 1.0 | 2.1 | RI, MS, 13C-NMR |
| 59 | β-Elemene | 1387 | 1589 | 0.751 | - | 0.5 | 0.3 | RI, MS, 13C-NMR |
| 60 | (E)-β-Caryophyllene | 1417 | 1596 | 0.751 | - | 0.1 | 0.2 | RI, MS, ¹³C-NMR |
| 61 | Thujopsene | 1428 | 1617 | 0.751 | - | 0.2 | 0.3 | RI, MS, ¹³C-NMR |
| 62 | Prezizaene | 1444 | 1630 | 0.751 | - | 0.1 | 0.1 | RI, MS |
| 63 | α-Humulene | 1447 | 1667 | 0.751 | - | tr | tr | RI, MS |
| 64 | Selina-4,11-diene | 1470 | 1670 | 0.751 | - | tr | tr | RI, MS |
| 65 | β-Selinene | 1481 | 1712 | 0.751 | - | 0.4 | 0.3 | RI, MS, ¹³C-NMR |
| 66 | α-Selinene | 1490 | 1718 | 0.751 | - | 0.3 | 0.2 | RI, MS, ¹³C-NMR |
| 67 | β-Bisabolene | 1500 | 1720 | 0.751 | - | 0.1 | 0.2 | RI, MS, ¹³C-NMR |
| 68 | γ-Cadinene | 1506 | 1750 | 0.751 | - | tr | 0.1 | RI, MS |
| 69 | γ-Cuprenene | 1523 | - | 0.751 | - | - | tr | RI, MS |
| 70 | β-Elemol | 1534 | 2073 | 0.819 | - | 0.1 | tr | RI, MS |
| 71 | Caryophyllene oxide | 1569 | 1981 | 0.830 | - | tr | 0.1 | RI, MS |
| 72 | Cedrol | 1588 | 2105 | 0.819 | 0.2 | 0.6 | 1.1 | RI, MS, 13C-NMR |
| 73 | γ-Eudesmol | 1617 | 2158 | 0.819 | - | tr | - | RI, MS |
| 74 | T-Cadinol | 1625 | 2163 | 0.819 | - | - | tr | RI, MS |
| 75 | β-Eudesmol | 1634 | 2218 | 0.819 | - | 0.1 | tr | RI, MS |
| 76 | Eudesm-11-en-4α-ol | 1637 | - | 0.819 | - | - | 0.2 | RI, MS |
| 77 | α-Eudesmol | 1639 | 2209 | 0.819 | - | 0.1 | tr | RI, MS |
| 78 | (Z)-Heptadec-8-ene | 1676 | - | 0.723 | - | - | tr | RI, MS |
| 79 | Manool oxide | 1983 | 2334 | 0.795 | - | 0.1 | tr | RI, MS |
| 80 | (E)-Biformene | 2003 | 2377 | 0.744 | - | 0.2 | 0.1 | RI, MS |
| 81 | Abietatriene | 2035 | 2486 | 0.751 | - | 0.3 | 0.1 | RI, MS |
| 82 | Sandaracopimarinal | 2157 | 2789 | 0.810 | - | 0.2 | 0.1 | RI, MS |
| 83 | Dehydroabietal | 2222 | - | 0.774 | - | 0.3 | 0.1 | RI, MS |
| Total | 93.8 | 99.8 | 89.9 |
| C | 13C δ (ppm) | 1H | 1H δ (ppm) by HSQC | COSY 1H-1H | HMBC H → C | NOESY a |
|---|---|---|---|---|---|---|
| 1 | 43.02 | 1 | 1.96 (td, 3J, 4J = 5.8 Hz; 4J = 1.2 Hz) | 3, 4b, 5, 7b | 2, 3, 5, 6, 7, 8, 10 | 5, 8a, 8b, 10 |
| 2 | 144.08 | - | - | - | - | - |
| 3 | 117.22 | 3 | 5.21 (m, J = 1.5 Hz) | 1, 4a, 4b, 10 | 1, 5, 10 | 4a, 4b, 9, 10 |
| 4 | 31.18 | 4a (anti) | 2.21 (dm, 2J = 17.1 Hz; J = 2.4 Hz) | 3, 4b, 5, 10 | - | 3, 4b, 5, 7a |
| 4b (syn) | 2.08 (dm, 2J = 17.1 Hz; J = 2.4 Hz) | 1, 3, 4a, 5, 10 | 2, 3 | 3, 4a, 5, 9 | ||
| 5 | 36.72 | 5 | 2.06 (m) | 1, 4a, 4b, 7b | 1 | 1, 4a, 4b, 7b, 8a, 8b |
| 6 | 43.75 | - | - | - | - | - |
| 7 | 31.88 | 7a (endo) | 1.23 (d, 2J = 8.7 Hz) | 7b | 1, 2, 4, 5, 6, 9 | 4a, 7b |
| 7b (exo) | 2.16 (dt, 2J = 8.7 Hz; 3J = 5.8 Hz) | 1, 5, 7a | 1, 2, 4, 5 | 5, 7a, 8a, 8b | ||
| 8 | 69.00 | 8a | 3.54 (d, 2J = 10.6 Hz) | 8b, 9 | 1, 5, 9 | 1, 5, 7b, 9 |
| 8b | 3.50 (d, 2J = 10.6 Hz) | 8a, 9 | 1, 5, 9 | 1, 5, 7b, 9 | ||
| 9 | 16.03 | 9 | 0.95 (s) | 8a, 8b | 1, 5, 6, 8 | 3, 4b, 8a, 8b, 10 |
| 10 | 23.06 | 10 | 1.61 (m, J = 1.9 Hz) | 3, 4a, 4b | 1, 2, 3 | 1, 3, 9 |
| C | 13C δ (ppm) | 1H | 1H δ (ppm) by HSQC | COSY 1H-1H | HMBC H → C | NOESY a |
|---|---|---|---|---|---|---|
| 1 | 43.28 | 1 | 1.98 (td, 3J, 4J = 5.7 Hz; 4J = 1.2 Hz) | 3, 5, 7b | 2, 3, 5, 8, 10 | 5, 7a, 7b, 8a, 8b |
| 2 | 143.57 | - | - | - | - | - |
| 3 | 117.29 | 3 | 5.17 (m, J = 1.5 Hz) | 1, 4a, 4b, 10 | - | 4b, 9, 10 |
| 4 | 30.94 | 4a (anti) | 2.16 (dm, 2J = 17.4 Hz; J = 2.5 Hz) | 3, 4b, 5, 10 | - | 4b, 10 |
| 4b (syn) | 2.03 (dm, 2J = 17.4 Hz; J = 2.5 Hz) | 3, 4a, 5, 10 | - | 3, 4a, 5, 9, 10 | ||
| 5 | 37.08 | 5 | 2.10 (m) | 1, 4a, 4b, 7b | - | 1, 4b, 7b, 8a, 8b, 12 |
| 6 | 41.99 | - | - | - | - | - |
| 7 | 31.73 | 7a (endo) | 1.21 (d, 2J = 8.9 Hz) | 7b | 1, 2, 4, 5, 6, 9 | 1, 7b |
| 7b (exo) | 2.25 (dt, 2J = 8.9 Hz; 3J = 5.7 Hz) | 1, 5, 7a | 1, 2, 4, 5 | 1, 5, 7a, 8a, 8b, 12 | ||
| 8 | 70.58 | 8a | 4.32 (d, 2J = 11.1 Hz) | 8b, 9 | 1, 5, 6, 9, 11 | 1, 5, 7b, 9 |
| 8b | 4.26 (d, 2J = 11.1 Hz) | 8a, 9 | 1, 5, 6, 9, 11 | 1, 5, 7b, 9 | ||
| 9 | 16.40 | 9 | 0.96 (s) | 8a, 8b | 1, 5, 6, 8 | 3, 4b, 8a, 8b, 12 |
| 10 | 22.95 | 10 | 1.57 (m, J = 1.9 Hz) | 3, 4a, 4b | 1, 2, 3, 4 | 3, 4a, 4b |
| 11 | 170.48 | - | - | - | - | - |
| 12 | 20.53 | 12 | 1.72 (s) | - | 8, 11 | 5, 7b, 9 |
| C | 13C δ (ppm) of C | 1H of C | 1H δ (ppm) by HSQC of C | 13C δ (ppm) of E |
|---|---|---|---|---|
| 1 | 42.86 | 1 | 2.29 (td, 3J, 4J = 5.7 Hz; 4J = 1.4 Hz) | 45.31 |
| 2 | 142.26 | - | - | 142.08 |
| 3 | 117.99 | 3 | 5.12 (m, J = 1.6 Hz) | 117.21 |
| 4 | 30.27 | 4a (anti) | 2.08 (dm, 2J = 17.8 Hz; J = 2.5 Hz) | 30.25 |
| 4b (syn) | 1.85 (dm, 2J = 17.8 Hz; J = 2.5 Hz) | |||
| 5 | 36.77 | 5 | 2.40 (md, 4J = 1.2 Hz) | 39.35 |
| 6 | 53.25 | - | - | 49.14 |
| 7 | 30.76 | 7a (endo) | 1.10 (d, 2J = 8.7 Hz) | 32.06 |
| 7b (exo) | 2.06 (dt, 2J = 8.7 Hz; 3J = 5.7 Hz) | |||
| 8 | 204.19 | 8 | 9.62 (s) | 184.91 |
| 9 | 12.24 | 9 | 0.73 (s) | 16.20 |
| 10 | 22.70 | 10 | 1.49 (m, J = 2.0 Hz) | 22.77 |
| C | 13C δ (ppm) | 1H | 1H δ (ppm) by HSQC | COSY 1H-1H | HMBC H → C | NOESY a |
|---|---|---|---|---|---|---|
| 1 | 45.44 | 1 | 2.77 (td, 3J, 4J = 5.6 Hz; 4J = 1.4 Hz) | 3, 5, 7b | 2, 3, 5, 6, 7, 8, 10 | 5, 7b, 10 |
| 2 | 142.22 | - | - | - | - | - |
| 3 | 117.19 | 3 | 5.14 (m, J = 1.5 Hz) | 1, 4a, 4b, 10 | - | 4a, 4b, 9, 10 |
| 4 | 30.32 | 4a (anti) | 2.15 (dm, 2J = 17.6 Hz; J = 2.4 Hz) | 3, 4b, 5, 10 | 1, 2, 5, 6 | 3, 4b, 7a, 10 |
| 4b (syn) | 2.03 (dm, 2J = 17.6 Hz; J = 2.4 Hz) | 3, 4a, 5, 10 | 2, 3, 5, 6, 7 | 3, 4a, 9, 10 | ||
| 5 | 39.46 | 5 | 2.87 (md, 4J = 1.2 Hz) | 1, 4a, 4b, 7b | - | 1, 7b |
| 6 | 49.05 | - | - | - | - | - |
| 7 | 32.05 | 7a (endo) | 1.23 (d, 2J = 8.6 Hz) | 7b | 1, 2, 4, 5, 6, 9 | 4a, 7b |
| 7b (exo) | 2.16 (dt, 2J = 8.6 Hz; 3J = 5.6 Hz) | 1, 5, 7a | 1, 2, 4, 5, 6 | 1, 5, 7a, 11 | ||
| 8 | 178.28 | - | - | - | - | - |
| 9 | 16.24 | 9 | 1.11 (s) | - | 1, 5, 6, 8 | 3, 4b, 10 |
| 10 | 22.85 | 10 | 1.57 (m, J = 1.9 Hz) | 3, 4a, 4b | 1, 2, 3 | 1, 3, 4a, 4b, 9 |
| 11 | 51.47 | 11 | 3.43 (s) | - | 8 | 7b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, G.; Tissandié, L.; Filippi, J.-J.; Tomi, F. New Pinane Derivatives Found in Essential Oils of Calocedrus decurrens. Molecules 2017, 22, 921. https://doi.org/10.3390/molecules22060921
Garcia G, Tissandié L, Filippi J-J, Tomi F. New Pinane Derivatives Found in Essential Oils of Calocedrus decurrens. Molecules. 2017; 22(6):921. https://doi.org/10.3390/molecules22060921
Chicago/Turabian StyleGarcia, Gabriel, Loïc Tissandié, Jean-Jacques Filippi, and Félix Tomi. 2017. "New Pinane Derivatives Found in Essential Oils of Calocedrus decurrens" Molecules 22, no. 6: 921. https://doi.org/10.3390/molecules22060921
APA StyleGarcia, G., Tissandié, L., Filippi, J.-J., & Tomi, F. (2017). New Pinane Derivatives Found in Essential Oils of Calocedrus decurrens. Molecules, 22(6), 921. https://doi.org/10.3390/molecules22060921