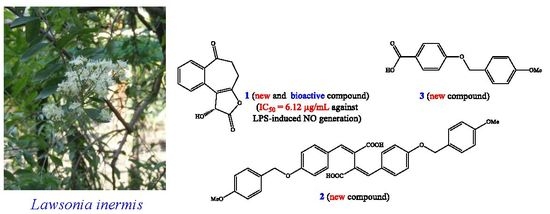
New Benzenoid Derivatives and Other Constituents from Lawsonia inermis with Inhibitory Activity against NO Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation
2.2. Structure Identification of the Known Isolates
2.3. Inhibitory Activity against Nitric Oxide Production
3. Experimental Section
3.1. General
3.2. Chemicals
3.3. Plant Material
3.4. Extraction and Isolation
3.5. Cell Culture
3.6. Cell Viability
3.7. Measurement of Nitric Oxide/Nitrite
3.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, H.Y.; Qian, C. Flora of China; Editorial Committee of the Flora of China: Beijing, China, 2007; Volume 13, pp. 274–288. [Google Scholar]
- Lin, Y.X.; Chang, Y.S.; Chen, I.S.; Ou, J.C. The Catalogue of Medicinal Plant. Resources in Taiwan; Committee on Chinese Medicine and Pharmacy: Taipei, Taiwan, 2003; p. 333. [Google Scholar]
- Yang, C.S.; Huang, H.C.; Wang, S.Y.; Sung, P.J.; Huang, G.J.; Chen, J.J.; Kuo, Y.H. New diphenol and isocoumarins from the aerial part of Lawsonia inermis and their inhibitory activities against NO production. Molecules 2016, 21, 1299. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rahman, A.; Alam, A.; Saleem, M.; Athar, M.; Sultana, S. Evaluation of the efficacy of Lawsonia alba in the alleviation of carbon tetrachloride-induced oxidative stress. J. Ethnopharmacol. 2000, 69, 157–164. [Google Scholar] [CrossRef]
- Liou, J.R.; Mohamed, E.S.; Du, Y.C.; Tseng, C.N.; Hwang, T.L.; Chuang, Y.L.; Hsu, Y.M.; Hsieh, P.W.; Wu, C.C.; Chen, S.L.; et al. 1,5-Diphenylpent-3-en-1-ynes and methyl naphthalene carboxylates from Lawsonia inermis and their anti-inflammatory activity. Phytochemistry 2013, 88, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.D.; Subhasree, R.S. Antimicrobial activities of Lawsonia inermis—A review. Acad. J. Plant Sci. 2009, 2, 231–232. [Google Scholar]
- Barluenga, J.; Aznar, F.; Gutiérrez, I.; Martín, J.A. Cyclopropanation with Fischer acyloxycarbene complexes: Preparation of cyclopropane and cycloheptane-fused γ-lactones. Org. Lett. 2002, 4, 2719–2722. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.J.; Paull, D.H.; Bekele, T.; Scerba, M.T.; Dudding, T.; Lectka, T. A surprising mechanistic “switch” in Lewis acid activation: A bifunctional, asymmetric approach to α-hydroxy acid derivatives. J. Am. Chem. Soc. 2008, 130, 17085–17094. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Yang, S.M. Total synthesis of (+)-brasilenyne. Application of an intramolecular silicon-assisted cross-coupling reaction. J. Am. Chem. Soc. 2004, 126, 12432–12440. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wen, Y. Synthesis of threo-(±)-9, 9-dibenzoylsecoisolariciresinol and its isomer. J. Chem. Res. 2010, 11, 606–609. [Google Scholar] [CrossRef]
- Elbe, H.L.; Köbrich, G. Darstellung und Konfiguration der drei isomeren Dibenzylidenbernsteinsäuren (α, δ-Diphenylfulgensäuren). Chem. Ber. 1974, 107, 1654–1666. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, H.Y.; Kim, Y.S.; Seo, J.H.; Roh, E.J.; Han, H.; Shin, K.J. Small molecules that protect against β-amyloid-induced cytotoxicity by inhibiting aggregation of β-amyloid. Bioorg. Med. Chem. 2012, 20, 4921–4935. [Google Scholar] [CrossRef] [PubMed]
- Mosley, C.A. Design, Synthesis, and Biological Evaluation of Novel N-methyl-d-aspartate Receptor Antagonists. Ph.D. Thesis, Emory University, Atlanta, GA, USA, 2009; p. 383. [Google Scholar]
- Schmidt, B.; Hölter, F.; Berger, R.; Jessel, S. Mizoroki-Heck Reactions with 4-Phenoldiazonium Salts. Adv. Synth. Catal. 2010, 352, 2463–2473. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, J.R.; Tsai, F.Y. Palladium(II)/cationic 2,2′-bipyridyl system as a highly efficient and reusable catalyst for the Mizoroki-Heck reaction in water. Molecules 2010, 15, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Prevost, M.S.; Cochin, S.D.; Marteaux, J.; Colas, C.; Renterghem, C.V.; Blondel, A.; Malliavin, T.; Corringer, P.J.; Joseph, D. Identification of cinnamic acid derivatives as novel antagonists of the prokaryotic proton-gated ion channel GLIC. J. Med. Chem. 2013, 56, 4619–4630. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, F.L.S.; Silva, M.M.P.; Alves, W.A.; Pinheiro, C.B.; Resende, J.A.L.C.; Lanznaster, M. Isomerism and nuclearity control in bis(lawsonato)zinc(II) complexes. Polyhedron 2012, 42, 43–49. [Google Scholar] [CrossRef]
- Moss, R.J.; White, R.O.; Rickborn, B. α,α-Dimethoxy-o-xylylene (5-(dimethoxymethylene)-6-methylene-1,3-cyclohexadiene): Formation by 1,4-elimination and electrocyclic routes and reactions. J. Org. Chem. 1985, 50, 5132–5139. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, Y.Y.; Xu, Y.N.; Dai, L.Y. Ring-opening of cyclic anhydrides using ionic liquids. J. Chem. Res. 2009, 5, 167–169. [Google Scholar] [CrossRef]
- Parida, K.N.; Moorthy, J.N. Synthesis of o-carboxyarylacrylic acids by room temperature oxidative cleavage of hydroxynaphthalenes and higher aromatics with oxone. J. Org. Chem. 2015, 80, 8354–8360. [Google Scholar] [CrossRef] [PubMed]
- Chiarotto, I.; Feroci, M.; Sotgiu, G.; Inesi, A. The dual role of ionic liquid BmimBF4, precursor of N-heterocyclic carbene and solvent, in the oxidative esterification of aldehydes. Tetrahedron 2013, 69, 8088–8095. [Google Scholar] [CrossRef]
- Hossain, M.M.; Shyu, S.G. Biphasic copper-catalyzed C-H bond activation of arylalkanes to ketones with tert-butyl hydroperoxide in water at room temperature. Tetrahedron 2016, 72, 4252–4257. [Google Scholar] [CrossRef]
- Geller, D.A.; Billiar, T.R. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998, 17, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmcol. Rev. 1991, 43, 109–142. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

 ) and HMBC (
) and HMBC (  ) correlations of 1.
) correlations of 1.
 ) and HMBC (
) and HMBC (  ) correlations of 2.
) correlations of 2.
 ) and HMBC (
) and HMBC (  ) correlations of 3.
) correlations of 3.
| Compounds | IC50 (μg/mL) a |
|---|---|
| 1 | 6.12 ± 2.84 |
| 2 | >20 |
| 3 | >20 |
| 4 | 16.43 ± 2.68 |
| 5 | 18.98 ± 3.48 |
| 6 | 9.30 ± 4.26 |
| 7 | >20 |
| 8 | >20 |
| 9 | >20 |
| 10 | >20 |
| 11 | >20 |
| 12 | >20 |
| 13 | 9.30 ± 4.68 |
| 14 | 14.90 ± 3.86 |
| Indomethacin b | 59.48 ± 1.22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-S.; Chen, J.-J.; Huang, H.-C.; Huang, G.-J.; Wang, S.-Y.; Sung, P.-J.; Cheng, M.-J.; Wu, M.-D.; Kuo, Y.-H. New Benzenoid Derivatives and Other Constituents from Lawsonia inermis with Inhibitory Activity against NO Production. Molecules 2017, 22, 936. https://doi.org/10.3390/molecules22060936
Yang C-S, Chen J-J, Huang H-C, Huang G-J, Wang S-Y, Sung P-J, Cheng M-J, Wu M-D, Kuo Y-H. New Benzenoid Derivatives and Other Constituents from Lawsonia inermis with Inhibitory Activity against NO Production. Molecules. 2017; 22(6):936. https://doi.org/10.3390/molecules22060936
Chicago/Turabian StyleYang, Chang-Syun, Jih-Jung Chen, Hui-Chi Huang, Guan-Jhong Huang, Sheng-Yang Wang, Ping-Jyun Sung, Ming-Jen Cheng, Ming-Der Wu, and Yueh-Hsiung Kuo. 2017. "New Benzenoid Derivatives and Other Constituents from Lawsonia inermis with Inhibitory Activity against NO Production" Molecules 22, no. 6: 936. https://doi.org/10.3390/molecules22060936
APA StyleYang, C. -S., Chen, J. -J., Huang, H. -C., Huang, G. -J., Wang, S. -Y., Sung, P. -J., Cheng, M. -J., Wu, M. -D., & Kuo, Y. -H. (2017). New Benzenoid Derivatives and Other Constituents from Lawsonia inermis with Inhibitory Activity against NO Production. Molecules, 22(6), 936. https://doi.org/10.3390/molecules22060936