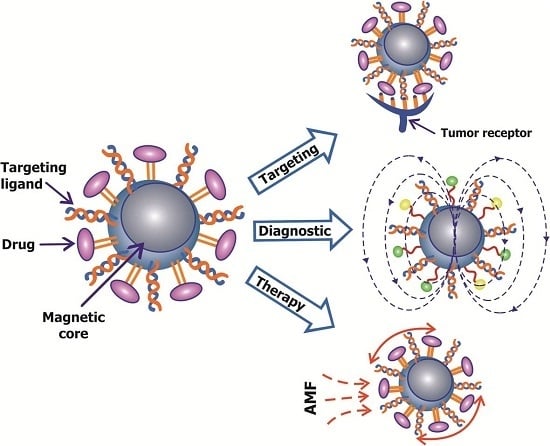
Targeted Magnetic Nanotheranostics of Cancer
Abstract
:1. Introduction
2. Aptamers
3. Characterization of Magnetic Nanoparticles
4. Production of Magnetic Nanoparticles
5. Coatings and Functionalization of MNPs
6. Application in Diagnostics
7. Magnetic Resonance Imaging
8. Application in Therapy
8.1. Chemotherapy or Drug Delivery
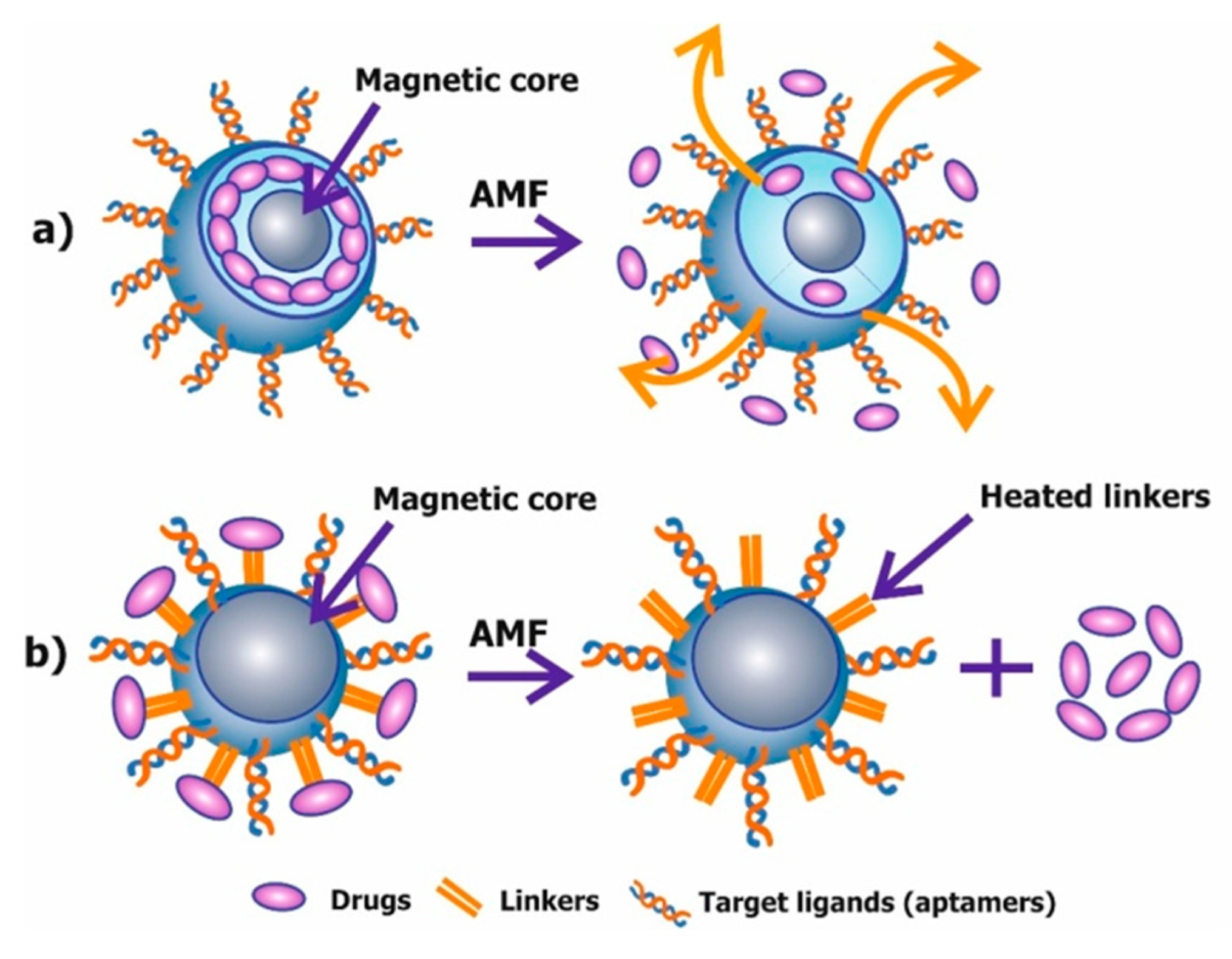
8.2. Magnetic Mediated Hyperthermia
8.3. Mechanical Destruction of Cells and Triggering of Apoptosis
9. Biodistribution and Toxicity of Gold and Magnetic Nanoparticles
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Veiseh, O.; Gunn, J.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Beqa, L.; Fan, Z.; Singh, A.; Senapati, D.; Ray, P. Gold nano-popcorn attached swcnt hybrid nanomaterial for targeted diagnosis and photothermal therapy of human breast cancer cells. ACS Appl. Mater. Interfaces 2011, 3, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Brewer, E.; Coleman, J.; Lowman, A. Emerging technologies of polymeric nanoparticles in cancer drug delivery. J. Nanomater. 2011. [Google Scholar] [CrossRef]
- Prabhu, R.; Patravale, V.; Joshi, M. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015, 10, 1001–1018. [Google Scholar]
- Ho, D.; Sun, X.; Sun, S. Monodisperse magnetic nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Ridi, F.; Bonini, M.; Baglioni, P. Magneto-responsive nanocomposites: Preparation and integration of magnetic nanoparticles into films, capsules, and gels. Adv. Colloid Interface Sci. 2014, 207, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Zhong, Y.; Cui, Y. Remote-controlled DNA release from fe3o4@au nanoparticles using an alternating electromagnetic field. J. Biomed. Nanotechnol. 2015, 11, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lian, Y.; Zhang, L.; Aldousari, S.; Hedia, H.; Asiri, S.; Liu, W. Cell and nanoparticle transport in tumour microvasculature: The role of size, shape and surface functionality of nanoparticles. Interface Focus 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Yu, J.; Hou, Y. Surface modification of magnetic nanoparticles in biomedicine. Chin. Phys. B 2015, 24, 014704. [Google Scholar] [CrossRef]
- Hajba, L.; Guttman, A. The use of magnetic nanoparticles in cancer theranostics: Toward handheld diagnostic devices. Biotechnol. Adv. 2016, 34, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.; Kulkarni, S.; Nacev, A.; Muro, S.; Stepanov, P.; Weinberg, I. Open challenges in magnetic drug targeting. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, T.; Zhao, J.; Huang, Y.; Deng, H.; Kumar, A.; Wang, C.; Liang, Z.; Ma, X.; Liang, X. Multifunctional aptamer-based nanoparticles for targeted drug delivery to circumvent cancer resistance. Biomaterials 2016, 91, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Chertok, B.; Moffat, B.; David, A.; Yu, F.; Bergemann, C.; Ross, B.; Yang, V. Iron oxide nanoparticles as a drug delivery vehicle for mri monitored magnetic targeting of brain tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Aarntzen, E.; Bulte, J.; Oyen, W.; Heerschap, A.; de Vries, I.; Figdor, C. Imaging of cellular therapies. Adv. Drug Deliv. Rev. 2010, 62, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.; Moore, A.; Medarova, Z. Magnetic nanoparticles for cancer diagnosis and therapy. Pharm. Res. 2012, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yan, C.; Yan, Y.; Chen, L.; Song, L.; Zang, F.; An, Y.; Teng, G.; Gu, N.; Zhang, Y. Multi-modal mn-zn ferrite nanocrystals for magnetically-induced cancer targeted hyperthermia: A comparison of passive and active targeting effects. Nanoscale 2016, 8, 16902–16915. [Google Scholar] [CrossRef] [PubMed]
- Gersting, S.; Schillinger, U.; Lausier, J.; Nicklaus, P.; Rudolph, C.; Plank, C.; Reinhardt, D.; Rosenecker, J. Gene delivery to respiratory epithelial cells by magnetofection. J. Gene Med. 2004, 6, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.T.; Shah, B.P.; Lee, K.B. Combined magnetic nanoparticle-based microrna and hyperthermia therapy to enhance apoptosis in brain cancer cells. Small 2014, 10, 4106–4112. [Google Scholar] [CrossRef] [PubMed]
- Latorre, A.; Couleaud, P.; Aires, A.; Cortajarena, A.; Somoza, A. Multifunctionalization of magnetic nanoparticles for controlled drug release: A general approach. Eur. J. Med. Chem. 2014, 82, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Stroberg, W.; Lee, T.; Kim, H.; Man, H.; Ho, D.; Decuzzi, P.; Liu, W. Multiscale modeling and uncertainty quantification in nanoparticle-mediated drug/gene delivery. Comput. Mech. 2014, 53, 511–537. [Google Scholar] [CrossRef]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dahring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Yusof, N. Doxorubicin-loaded magnetic gold nanoshells for a combination therapy of hyperthermia and drug delivery. J. Colloid Interface Sci. 2014, 434, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Pala, K.; Serwotka, A.; Jelen, F.; Jakimowicz, P.; Otlewski, J. Tumor-specific hyperthermia with aptamer-tagged superparamagnetic nanoparticles. Int. J. Nanomed. 2014, 9, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Pasquale, N.; De, G.; Tan, T.; Ma, J.; Lee, K. Core-shell nanoparticle-based peptide therapeutics and combined hyperthermia for enhanced cancer cell apoptosis. ACS Nano 2014, 8, 9379–9387. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhu, Y. Preparation of magnetic mesoporous silica nanoparticles as a multifunctional platform for potential drug delivery and hyperthermia. Sci. Technol. Adv. Mater. 2016, 17, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sahoo, S. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Lee, J.; Shin, T.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Lu, P.; Rosato, R.; Tan, W.; Zu, Y. Oligonucleotide aptamers: New tools for targeted cancer therapy. Mol. Ther. Nucleic Acids 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Zamay, A.S.; Kolovskaya, O.S.; Reshetneva, I.T.; Zamay, G.S.; Kibbee, R.J.; Sattar, S.A.; Zamay, T.N.; Berezovski, M.V. Aptamer-based impedimetric sensor for bacterial typing. Anal. Chem. 2012, 84, 8114–8117. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Zamay, A.S.; Muharemagic, D.; Chechik, A.V.; Bell, J.C.; Berezoyski, M.V. Aptamer-based viability impedimetric sensor for viruses. Anal. Chem. 2012, 84, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, Z.; An, Y.; Zhang, W.; Zhang, H.; Liu, D.; Yu, C.; Duan, W.; Yang, C. Selection of DNA aptamers against epithelial cell adhesion molecule for cancer cell imaging and circulating tumor cell capture. Anal. Chem. 2013, 85, 4141–4149. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, M.; de Prada, P.; Landry, D. Aptamer-based folding fluorescent sensor for cocaine. J. Am. Chem. Soc. 2001, 123, 4928–4931. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, M.; Labib, M.; Muharemagic, D.; Zamay, A.; Berezovski, M. Switchable aptamers for biosensing and bioseparation of viruses (swaps-v). Biosens. Bioelectron. 2015, 67, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zamay, G.; Kolovskaya, O.; Zamay, T.; Glazyrin, Y.; Krat, A.; Zubkova, O.; Spivak, E.; Wehbe, M.; Gargaun, A.; Muharemagic, D.; et al. Aptamers selected to postoperative lung adenocarcinoma detect circulating tumor cells in human blood. Mol. Ther. 2015, 23, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Zamay, G.S.; Belyanina, I.V.; Zamay, A.S.; Komarova, M.A.; Krat, A.V.; Eremina, E.N.; Zukov, R.A.; Sokolov, A.E.; Zamay, T.N. Selection of DNA aptamers for breast cancer. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2016, 10, 158–164. [Google Scholar] [CrossRef]
- Zamay, G.S.; Zamay, T.N.; Kolovskii, V.A.; Shabanov, A.V.; Glazyrin, Y.E.; Veprintsev, D.V.; Krat, A.V.; Zamay, S.S.; Kolovskaya, O.S.; Gargaun, A.; et al. Electrochemical aptasensor for lung cancer-related protein detection in crude blood plasma samples. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Zamay, T.N.; Kolovskaya, O.S.; Glazyrin, Y.E.; Zamay, G.S.; Kuznetsova, S.A.; Spivak, E.A.; Wehbe, M.; Savitskaya, A.G.; Zubkova, O.A.; Kadkina, A.; et al. DNA-aptamer targeting vimentin for tumor therapy in vivo. Nucleic Acid Ther. 2014, 24, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Zimbres, F.; Tarnok, A.; Ulrich, H.; Wrenger, C. Aptamers: Novel molecules as diagnostic markers in bacterial and viral infections? Biomed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kruspe, S.; Mittelberger, F.; Szameit, K.; Hahn, U. Aptamers as drug delivery vehicles. Chemmedchem 2014, 9, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Bonnemain, B. Superparamagnetic agents in magnetic resonance imaging: Physicochemical characteristics and clinical applications—A review. J. Drug Target. 1998, 6, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Nieh, M.; Li, Y. Decorating nanoparticle surface for targeted drug delivery: Opportunities and challenges. Polymers 2016, 8, 83. [Google Scholar] [CrossRef]
- Yu, M.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Burgo, L.S.; Hernández, R.M.; Orive, G.; Pedraz, J.L. Nanotherapeutic approaches for brain cancer management. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V.; De, M.; Chu, S.; Jaiswal, M.; Rotz, M.; Meade, T.J.; Dravid, V.P. Theranostic magnetic nanostructures (mns) for cancer. Cancer Treat. Res. 2015, 166, 51–83. [Google Scholar] [PubMed]
- Nikiforov, V.N. Medical applications of magnetic nanoparticles. In Proceedings of the Academy of Engineering Sciences; Prokhorov, A.M., Ed.; Publisher: Moscow, Russia, 2013; pp. 23–34. [Google Scholar]
- Vitol, E.; Novosad, V.; Rozhkova, E. Microfabricated magnetic structures for future medicine: From sensors to cell actuators. Nanomedicine 2012, 7, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kohler, N.; Zhang, M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 2002, 23, 1553–1561. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Laurent, S.; Shokrgozar, M.; Hosseinkhani, M. Toxicity evaluations of superparamagnetic iron oxide nanoparticles: Cell "vision" versus physicochemical properties of nanoparticles. ACS Nano 2011, 5, 7263–7276. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Milani, A.; Stroeve, P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2009, 336, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Rozhkova, E.; Novosad, V.; Kim, D.; Pearson, J.; Divan, R.; Rajh, T.; Bader, S. Ferromagnetic microdisks as carriers for biomedical applications. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Reddy, L.; Arias, J.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, G.P.; Grishchenko, L.A.; Medvedeva, S.A.; Tikov, A.V.; Feoktistova, L.P.; Sapozhnikov, A.N.; Vakulskaya, T.I.; Tirsky, V.V.; Semenov, A.L.; Martynovich, B.; et al. Synthesis of nanosized particles with magnetic properties for biomedical purposes. Phys. Mesomech. 2004, 7, 139–142. [Google Scholar]
- Goon, I.; Lai, L.; Lim, M.; Munroe, P.; Gooding, J.; Amal, R. Fabrication and dispersion of gold-shell-protected magnetite nanoparticles: Systematic control using polyethyleneimine. Chem. Mater. 2009, 21, 673–681. [Google Scholar] [CrossRef]
- Corot, C.; Robert, P.; Idee, J.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Rawn, C.; Rondinone, A.; Love, L.; Roh, Y.; Everett, S.; Lauf, R.; Phelps, T. Large-scale production of magnetic nanoparticles using bacterial fermentation. J. Ind. Microbiol. Biotechnol. 2010, 37, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Roh, Y.; Lauf, R.; Vali, H.; Yeary, L.; Phelps, T. Microbial preparation of metal-substituted magnetite nanoparticles. J. Microbiol. Methods 2007, 70, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Prozorov, T.; Bazylinski, D.; Mallapragada, S.; Prozorov, R. Novel magnetic nanomaterials inspired by magnetotactic bacteria: Topical review. Mater. Sci. Eng. R Rep. 2013, 74, 133–172. [Google Scholar] [CrossRef]
- De, M.N.; Chu, S.; Jaiswal, M.; Rotz, M.; Meade, T.J.; Dravid, V.P. Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer; Springer International Publishing: Cham, Switzerland, 2015; Volume 166, pp. 51–83. [Google Scholar]
- Lee, H.; Shin, T.; Cheon, J.; Weissleder, R. Recent developments in magnetic diagnostic systems. Chem. Rev. 2015, 115, 10690–10724. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Chess, R. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Wei, X.; Li, N.; Lei, Z. In-situ formation of fe3o4 nanoparticles within the thermo sensitive hairy hybrid particles. Mater. Lett. 2008, 62, 2963–2966. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.; Zhang, M. Magnetic nanoparticles in mr imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Fortin-Ripoche, J.; Martina, M.; Gazeau, F.; Menager, C.; Wilhelm, C.; Bacri, J.; Lesieur, S.; Clement, O. Magnetic targeting of magnetoliposomes to solid tumors with mr imaging monitoring in mice: Feasibility. Radiology 2006, 239, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Salabas, E.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.M.; Tavallaie, R.; Sandiford, L.; Tilleya, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: Preparation, surface modification for analytical and biomedical applications. Int. J. Mol. Sci. 2013, 14, 6223–6240. [Google Scholar]
- Fattahi, H.; Laurent, S.; Liu, F.; Arsalani, N.; Elst, L.; Muller, R. Magnetoliposomes as multimodal contrast agents for molecular imaging and cancer nanotheragnostics. Nanomedicine 2011, 6, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Chandola, C.; Kalme, S.; Casteleijn, M.; Urtti, A.; Neerathilingam, M. Application of aptamers in diagnostics, drug-delivery and imaging. J. Biosci. 2016, 41, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Bugaj, A. Targeted photodynamic therapy—A promising strategy of tumor treatment. Photochem. Photobiol. Sci. 2011, 10, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shiao, Y.; Huang, Y. Release of photoactivatable drugs from plasmonic nanoparticles for targeted cancer therapy. ACS Nano 2011, 5, 7796–7804. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gao, X. Multilayer coating of gold nanorods for combined stability and biocompatibility. Phys. Chem. Chem. Phys. 2011, 13, 10028–10035. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, X.; He, X.; Wang, K.; Cui, W.; He, D.; Jia, X. Au@ag/au nanoparticles assembled with activatable aptamer probes as smart “nano-doctors” for image-guided cancer thermotherapy. Nanoscale 2014, 6, 8754–8761. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sefah, K.; Bamrungsap, S.; Chang, H.; Tan, W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir 2008, 24, 11860–11865. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; Bhaumik, J.; Karver, M.; Erdem, S.; Weissleder, R. Targeted nanoagents for the detection of cancers. Mol. Oncol. 2010, 4, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wei, W.; Tanaka, H.; Kohama, K.; Ma, G.; Dobashi, T.; Maki, Y.; Wang, H.; Bi, J.; Dai, S. A galactosamine-mediated drug delivery carrier for targeted liver cancer therapy. Pharmacol. Res. 2011, 64, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jeong, H.; Park, I.; Cho, C.; Moon, H.; Yu, D.; Bom, H.; Sohn, M.; Oh, I. Asialoglycoprotein receptor targeted gene delivery using galactosylated polyethylenimine-graft-poly(ethylene glycol): In vitro and in vivo studies. J. Control. Release 2005, 108, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Chen, L.; Gu, W.; Li, Y. Galactosylated poly(2-(2-aminoethyoxy)ethoxy)phosphazene/DNA complex nanoparticles: In vitro and in vivo evaluation for gene delivery. Biomacromolecules 2010, 11, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Long, N.; Aboagye, E. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem. Soc. Rev. 2013, 42, 7816–7833. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Young, J.; Nixon, A.; Drezek, R. Encapsulated fe3o4/ag complexed cores in hollow gold nanoshells for enhanced theranostic magnetic resonance imaging and photothermal therapy. Small 2014, 10, 3246–3251. [Google Scholar] [CrossRef] [PubMed]
- Na, H.; Song, I.; Hyeon, T. Inorganic nanoparticles for mri contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Roach, M.; Alberini, J.; Pecking, A.; Testori, A.; Verrecchia, F.; Soteldo, J.; Ganswindt, U.; Joyal, J.; Babich, J.; Witte, R.; et al. Diagnostic and therapeutic imaging for cancer: Therapeutic considerations and future directions. J. Surg. Oncol. 2011, 103, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Park, I.; Jeong, Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef] [PubMed]
- Weis, C.; Blank, F.; West, A.; Black, G.; Woodward, R.; Carroll, M.; Mainka, A.; Kartmann, R.; Brandl, A.; Bruns, H.; et al. Labeling of cancer cells with magnetic nanoparticles for magnetic resonance imaging. Magn. Reson. Med. 2014, 71, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic nanoparticles in cancer theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, K.; Lee, H.; Xu, C.; Hsu, A.; Peng, S.; Chen, X.; Sun, S. Ultrasmall c(rgdyk)-coated Fe3o4 nanoparticles and their specific targeting to integrin alpha(v)beta(3)-rich tumor cells. J. Am. Chem. Soc. 2008, 130, 7542–7543. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.; Stephen, Z.; Veiseh, O.; Arami, H.; Wang, T.; Lai, V.; Park, J.; Ellenbogen, R.; Disis, M.; Zhang, M. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional spions. ACS Nano 2012, 6, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, M.; Tong, X.; Sun, N.; Zhou, L.; Cao, Y.; Wang, J.; Zhang, H. Aptamer-modified temperature-sensitive liposomal contrast agent for mr imaging. Biomacromolecules 2015, 16, 2618–2623. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y. Drug targeting and tumor heterogeneity. J. Control. Release 2009, 133, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.; Yang, V.; David, A. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011, 29, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Jalalian, S.; Taghdisi, S.; Hamedani, N.; Kalat, S.; Lavaee, P.; ZandKarimi, M.; Ghows, N.; Jaafari, M.; Naghibi, S.; Danesh, N.; et al. Epirubicin loaded super paramagnetic iron oxide nanoparticle-aptamer bioconjugate for combined colon cancer therapy and imaging in vivo. Eur. J. Pharm. Sci. 2013, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tang, Z.; Zhou, Y.; Zhou, X.; Jin, Y.; Li, D.; Yang, Y.; Zhou, S. Magnetic micelles as a potential platform for dual targeted drug delivery in cancer therapy. Int. J. Pharm. 2012, 429, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.; Chen, H.; Wu, X.; Mao, H. Egfrviii antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef] [PubMed]
- Fazilati, M. Folate decorated magnetite nanoparticles: Synthesis and targeted therapy against ovarian cancer. Cell Biol. Int. 2014, 38, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, U.; Keskin, T.; Tansik, G.; Mutlu, P.; Yalcin, S.; Unsoy, G.; Yakar, A.; Khodadust, R.; Gunduz, G. Idarubicin-loaded folic acid conjugated magnetic nanoparticles as a targetable drug delivery system for breast cancer. Biomed. Pharmacother. 2014, 68, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, S.; Li, B.; Ren, X.; Li, S.; Mahounga, D.; Cui, S.; Gu, Y.; Achilefu, S. Folate-modified gold nanoclusters as near-infrared fluorescent probes for tumor imaging and therapy. Nanoscale 2012, 4, 6050–6064. [Google Scholar] [CrossRef] [PubMed]
- Al Faraj, A.; Shaik, A.; Shaik, A. Magnetic single-walled carbon nanotubes as efficient drug delivery nanocarriers in breast cancer murine model: Noninvasive monitoring using diffusion-weighted magnetic resonance imaging as sensitive imaging biomarker. Int. J. Nanomed. 2015, 10, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.; von Maltzahn, G.; Harris, T.; Duza, T.; Vecchio, K.; Ruoslahti, E.; Bhatia, S. Remotely triggered release from magnetic nanoparticles. Adv. Mater. 2007, 19, 3932–3936. [Google Scholar] [CrossRef]
- Brazel, C. Magnetothermally-responsive nanomaterials: Combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release. Pharm. Res. 2009, 26, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, C.; Schmid, R.; Jurgons, R.; Kremer, M.; Wanner, G.; Bergemann, C.; Huenges, E.; Nawroth, T.; Arnold, W.; Parak, F. Targeting cancer cells: Magnetic nanoparticles as drug carriers. Eur. Biophy. J. Biophys. Lett. 2006, 35, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; An, X. Controllable release from magnetoliposomes by magnetic stimulation and thermal stimulation. Colloids Surf. B Biointerfaces 2013, 104, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Xiao, D.; Bose, A.; Deng, R.; Bothun, G. Low-dose chemotherapy of hepatocellular carcinoma through triggered-release from bilayer-decorated magnetoliposomes. Colloids Surf. B Biointerfaces 2014, 116, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Imai, Y.; Koumoto, K.; Kaiden, T.; Kono, K.; Aoshima, S. Magnetoresponsive on-demand release of hybrid liposomes formed from fe3o4 nanoparticles and thermosensitive block copolymers. Small 2011, 7, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.; Wydra, R.; Stocke, N.; Anderson, K.; Hilt, J. Magnetic nanoparticles and nanocomposites for remote controlled therapies. J. Control. Release 2015, 219, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Fouriki, A.; Farrow, N.; Clements, M.A.; Dobson, J. Evaluation of the magnetic field requirements for nanomagnetic gene transfection. Nano Rev. 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Prosen, L.; Hudoklin, S.; Cemazar, M.; Stimac, M.; Tratar, U.; Ota, M.; Scancar, J.; Romih, R.; Sersa, G. Magnetic field contributes to the cellular uptake for effective therapy with magnetofection using plasmid DNA encoding against mcam in b16f10 melanoma in vivo. Nanomedicine 2016, 11, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Yu, J.; Chen, Y.; Chan, K. Assembly of polyethylenimine-functionalized iron oxide nanoparticles as agents for DNA transfection with magnetofection technique. J. Mater. Chem. B 2014, 2, 7936–7944. [Google Scholar] [CrossRef]
- Xu, H.; Hou, Z.; Zhang, H.; Kong, H.; Li, X.; Wang, H.; Xie, W. An efficient trojan delivery of tetrandrine by poly(n-vinylpyrrolidone)-block-poly(epsilon-caprolactone) (pvp-b-pcl) nanoparticles shows enhanced apoptotic induction of lung cancer cells and inhibition of its migration and invasion. Int. J. Nanomed. 2014, 9, 231–242. [Google Scholar]
- Guo, L.; Huang, J.; Zheng, L. Bifunctional bacterial magnetic nanoparticles for tumor targeting. Nanoscale 2012, 4, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Naregalkar, R.; Vaidya, V.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Choo, E.; Ahmed, A.; Zhao, L.; Yang, Y.; Ramanujan, R.; Xue, J.; Di Fan, D.; Fan, H.; Ding, J. Magnetic nanoparticle-loaded polymer nanospheres as magnetic hyperthermia agents. J. Mater. Chem. B 2014, 2, 120–128. [Google Scholar] [CrossRef]
- Quinto, C.; Mohindra, P.; Tong, S.; Bao, G. Multifunctional superparamagnetic iron oxide nanoparticles for combined chemotherapy and hyperthermia cancer treatment. Nanoscale 2015, 7, 12728–12736. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Di Corato, R.; Lartigue, L.; Marangon, I.; Guardia, P.; Silva, A.; Luciani, N.; Clement, O.; Flaud, P.; Singh, J.; et al. Heat-generating iron oxide nanocubes: Subtle “destructurators” of the tumoral microenvironment. ACS Nano 2014, 8, 4268–4283. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Rozhkova, E.; Ulasov, I.; Bader, S.; Rajh, T.; Lesniak, M.; Novosad, V. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 2010, 9, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Jiang, S.; Chen, Z.; Zhao, W.; Yi, Y.; Yang, R.; Chen, B. Apoptosis selectively induced in bel-7402 cells by folic acid-modified magnetic nanoparticles combined with 100 hz magnetic field. Int. J. Nanomed. 2014, 9, 2043–2050. [Google Scholar]
- Kim, P.D.; Zamay, S.S.; Zamay, T.N.; Procopenko, V.S.; Kolovskaya, O.S.; Zamay, G.S.; Princ, V.Y.; Seleznev, V.A.; Komov, A.I.; Spivak, E.A.; et al. The antitumor effect of magnetic nanodiscs and DNA aptamer conjugates. Dokl. Biochem. Biophys. 2016, 466, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Lee, E.; Son, M.; Lee, J.; Yoo, D.; Kim, J.; Park, S.; Shin, J.; Cheon, J. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat. Mater. 2012, 11, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Zamay, T.N.; Zamay, G.S.; Belyanina, I.V.; Zamay, S.S.; Denisenko, V.V.; Kolovskaya, O.S.; Ivanchenko, T.I.; Grigorieva, V.L.; Garanzha, I.V.; Veprintsev, D.V.; et al. Noninvasive microsurgery using aptamer-functionalized magnetic microdiscs for tumor cell eradication. Nucleic Acid Ther. 2016, 27, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Belyanina, I.V.; Zamay, T.N.; Zamay, G.S.; Zamay, S.S.; Kolovskaya, O.S.; Ivanchenko, T.I.; Denisenko, V.V.; Kirichenko, A.K.; Glazyrin, Y.E.; Garanzha, I.V.; et al. In vivo cancer cells elimination guided by aptamer-functionalized gold-coated magnetic nanoparticles and controlled with low frequency alternating magnetic field. Theranostics 2017, in press. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Chen, Z.; Shin, D. Advances of cancer therapy by nanotechnology. Cancer Res. Treat. 2009, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]






| Materials | Size | Coatings | Antitumor Drugs | Linkers | Targeted Ligands | Applications | Reference |
|---|---|---|---|---|---|---|---|
| Gd-DTPA | 123.2 nm | Thermo-sensitive liposome (DPPC) | - | Carboxyl groups | Aptamers | MRI | [87] |
| Iron oxide (magnetite) | 51.43 ± 4.52 nm | - | Epirubicin | Amine, carboxyl groups | Aptamers | Targeted chemotherapy MRI | [90] |
| Iron oxide (magnetite) | 10 nm | - | Dextran | Thiol groups | Aptamers | Magnetic hyperthermia | [24] |
| Iron oxide (magnetite) | 12 ± 3 nm | - | Doxorubicin | Thiol groups | Aptamers | Magnetic hyperthermia computed tomography | [22] |
| Ironoxide (magnetite) | 15.4 nm | Gold Polyethylene-glycol | - | Amino and thiol groups | Aptamers | Targeted magnetic hyperthermia | [25] |
| Nickel magnetic microdisks | 500 nm | Gold | - | Thiol groups | Aptamers | Mechanical destruction of cells and triggering of apoptosis | [116,118] |
| Iron oxide (magnetite) | 50nm | Gold | - | Thiol groups | Aptamers | Apoptosis induction via fibronectin binding aptamers | [119] |
| Iron-tagged single-walled carbon nanotubes | 200–300 nm | Polyvinyl-pyrrolidone | Doxorubicin | Carboxyl groups | Antibodies | Targeted chemotherapy MRI | [96] |
| Ironoxide (magnetite) | 10 nm | Polyethylene-glycol | - | Carboxylate and Amino groups | Antibodies | Targeted therapy MRI | [92] |
| Ironoxide (magnetite) | 40 nm | Chitosan and polyethylene-glycol | - | Amino and thiol groups | Antibodies | MRI | [86] |
| Mn-Zn ferrite MNCs | 42.3nm | Polyethylene-glycol | - | Carboxyl groups | Сyclic tripeptide of arginine-glycine-aspartic acid | Targeted magnetic hyperthermia MRI | [17] |
| Ironoxide (magnetite) | 5 nm | Lipid bilayer (DPPC/PEG750-PE) | Doxorubicin | - | - | Targeted chemotherapy controlled by electromagnetic fields | [101] |
| Ironoxide (magnetite) | 6.8 nm | Gold | Doxorubicin | Cystmolecules | - | Chemotherapy magnetic hyperthermia combinatorial treatment | [23] |
| Iron oxide (magnetite, maghemite) | 16.1 nm | Mesoporous silica | Doxorubicin | - | - | Targeted chemotherapy and magnetic hyperthermia | [26] |
| Iron Oxide Nanocubes | 19 nm | Polyethylene-glycol | - | - | - | Magnetic hyperthermia MRI | [113] |
| Ironoxide (magnetite) | 14 nm | Phospholipid-Polyethylene-glycol coating | Doxorubicin | - | - | Chemotherapy-magnetic hyperthermia combinatorial treatment | [112] |
| Ironoxide (magnetite) | 5 nm | - | Cytostatic mitox-antrone | Phosphate groups | - | Targeted chemotherapy controlled by strong inhomogeneous magnetic field | [99] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belyanina, I.; Kolovskaya, O.; Zamay, S.; Gargaun, A.; Zamay, T.; Kichkailo, A. Targeted Magnetic Nanotheranostics of Cancer. Molecules 2017, 22, 975. https://doi.org/10.3390/molecules22060975
Belyanina I, Kolovskaya O, Zamay S, Gargaun A, Zamay T, Kichkailo A. Targeted Magnetic Nanotheranostics of Cancer. Molecules. 2017; 22(6):975. https://doi.org/10.3390/molecules22060975
Chicago/Turabian StyleBelyanina, Irina, Olga Kolovskaya, Sergey Zamay, Ana Gargaun, Tatiana Zamay, and Anna Kichkailo. 2017. "Targeted Magnetic Nanotheranostics of Cancer" Molecules 22, no. 6: 975. https://doi.org/10.3390/molecules22060975
APA StyleBelyanina, I., Kolovskaya, O., Zamay, S., Gargaun, A., Zamay, T., & Kichkailo, A. (2017). Targeted Magnetic Nanotheranostics of Cancer. Molecules, 22(6), 975. https://doi.org/10.3390/molecules22060975