2.2. Paracetamol Adsorption Test on Activated Carbons
Studying paracetamol adsorption on activated carbon is relevant because this compound is an emerging pollutant which conventional water treatment procedures do not effectively remove. Adsorption is a versatile technique, suitable for further exploration. The adsorption of pharmaceutical compounds from an aqueous phase on activated carbon was mainly due to interactions between the functional groups in the drug structure and the groups on the solid sorbent surface [
13]. In this work the effect of pH on the adsorbate-adsorbent interactions and on the distribution of these functional groups was investigated. These characteristics have significant effects on the adsorption capacity.
Weak electrolytes, such as paracetamol, coexist in both ionized (a base) and non-ionised (an acid) forms. The distribution of acid and basic forms is strictly dependent on the solution pH and their interaction with solid may favour or disfavour the adsorption process if forces of attraction or repulsion prevail, respectively.
The concentration of these conjugates depend on the solution pH and pKa (9.38 for paracetamol, see
Table 1) as expressed in the Henderson-Hasselbalch equation. From this equation, the amount of acidic and basic forms can be calculated. For example, 90% of paracetamol is in its protonated form up to pH 7, while in basic medium the phenol group proton is removed, and at pH 11, 90% of the deprotonated base is found. The presence of charges on the activated carbon surface corresponding to a specific solution pH is determined by pH
PZC. This represents the pH of the solution at which the net surface charge is neutral. Hence, at solution pH below this value, activated carbon surface has an overall positive charge. At solution pH higher than pH
PZC, the surface of the activated carbon becomes negatively charged, because of the deprotonation of functional groups [
14]. Considering all this, it is evident that solution pH exerts a significant influence on paracetamol adsorption on activated carbons.
Figure 1 shows the paracetamol adsorption isotherms on the three activated carbons at pH 7 and T = 298 K.
The adsorbed amount of paracetamol is the highest on GACr, followed by GAC and GACo. According to Fuentes et al. [
15], this result is due to the highest basicity of GACr associated with increased density of π-delocalized electrons generated during the heat treatment and with basic functional groups that, according to the results of the Boehm titration (
Table 2), are the highest for GACr.
Moreover, at pH 7, the pH
PZC of GACr is higher (cf.
Table 2), the surface is positively charged and the paracetamol is mainly in the neutral form, hence neither repulsion nor adsorption is favoured [
9].
Terzyk [
16] affirms that the adsorption of aromatic compounds from aqueous solution on activated carbon can occur according to three different mechanisms: dispersive interactions by π electrons, hydrogen bond formation and donor-acceptor electron complexes. Paracetamol adsorption on granular activated carbon is expected to involve all these mechanisms. However, the adsorption due to π electron interactions seems to be predominant because the oxygen functional groups capable of forming Lewis acid-base complexes or hydrogen bonds are lower on activated carbons with modified surface chemistry [
16].
On the contrary, GACo shows a lower adsorption capacity because it has a high concentration of acidic groups, the surface polarity is increased and hydrogen bond can be formed with water molecules, which have a greater proportion and higher polarity than paracetamol [
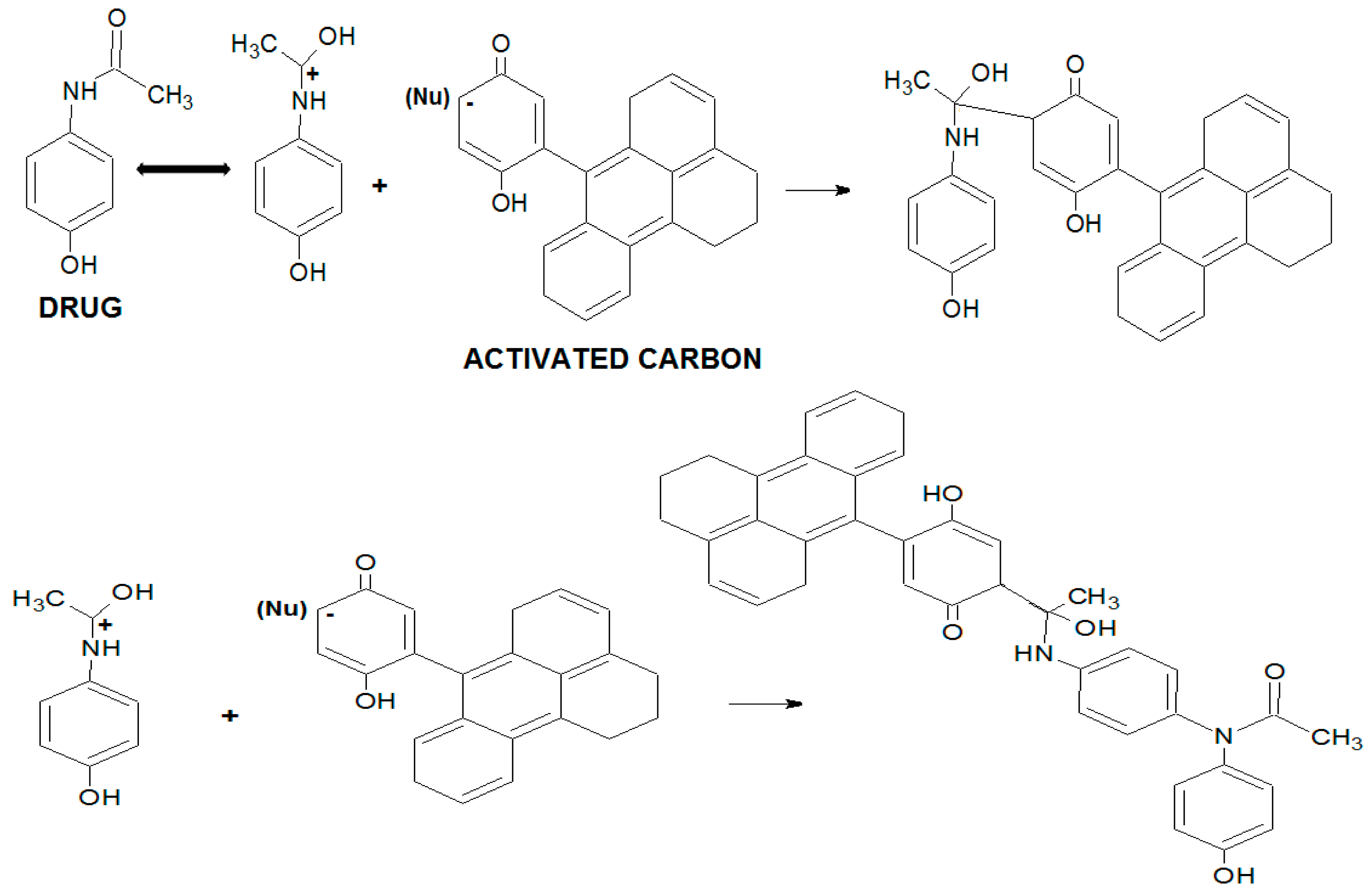
17]. The paracetamol molecule shows resonance structures of the free electron pair of the nitrogen atom, as shown in
Figure 2.
The formation of an electron acceptor-donor complex is possible between the groups with free electron pairs, such as oxygen of the phenol groups of activated carbons, and the electropositive nitrogen in paracetamol molecule, indicating that the decrease in the adsorption capacity can be related to the decline of these groups in the oxidized activated carbon. The paracetamol molecule has two proton acceptor and donor groups. However, due to the changes generated by the resonance, the nitrogen passes from proton acceptor to donor. Therefore, hydrogen bond interactions will be higher in activated carbon with higher concentrations of proton acceptors groups (i.e., GACr) [
16].
The π electron interactions are relevant for the adsorption of aromatic compounds. However, the influence exerted by the substituent type present in the aromatic molecule, such as paracetamol, should also be considered. In fact, the amide group present in paracetamol is an activating group of the aromatic ring, whereby π electrons in the molecule are available to generate interactions with the delocalised electrons of activated carbon.
As previously stated, the adsorption process of pharmaceutical compounds, such as paracetamol, is strictly influenced by electrostatic interactions between adsorbent and adsorbate. Therefore pH changes in the medium may generate changes in the adsorbate structure and activated carbon physicochemical properties. In acidic medium, the paracetamol molecule presents a nitrogen resonance electron pair, as shown in
Figure 2. The carbocation generation in the sp
2 carbon of the amide group facilitates the interaction with the proton acceptor groups of activated carbon, as well as nucleophilic addition with surface groups, such as hydrogen sulfides, trisubstituted amines and hydroxyls (
Figure 3) [
8,
18].
The nucleophilic addition reactions occur not only with activated carbon groups, in acidic medium. Paracetamol dimerization can also occur due to nucleophilic addition to the phenolic groups present in molecules of the same species [
19]. As the solution pH can exert a significant influence on paracetamol adsorption, a dedicated study was carried out.
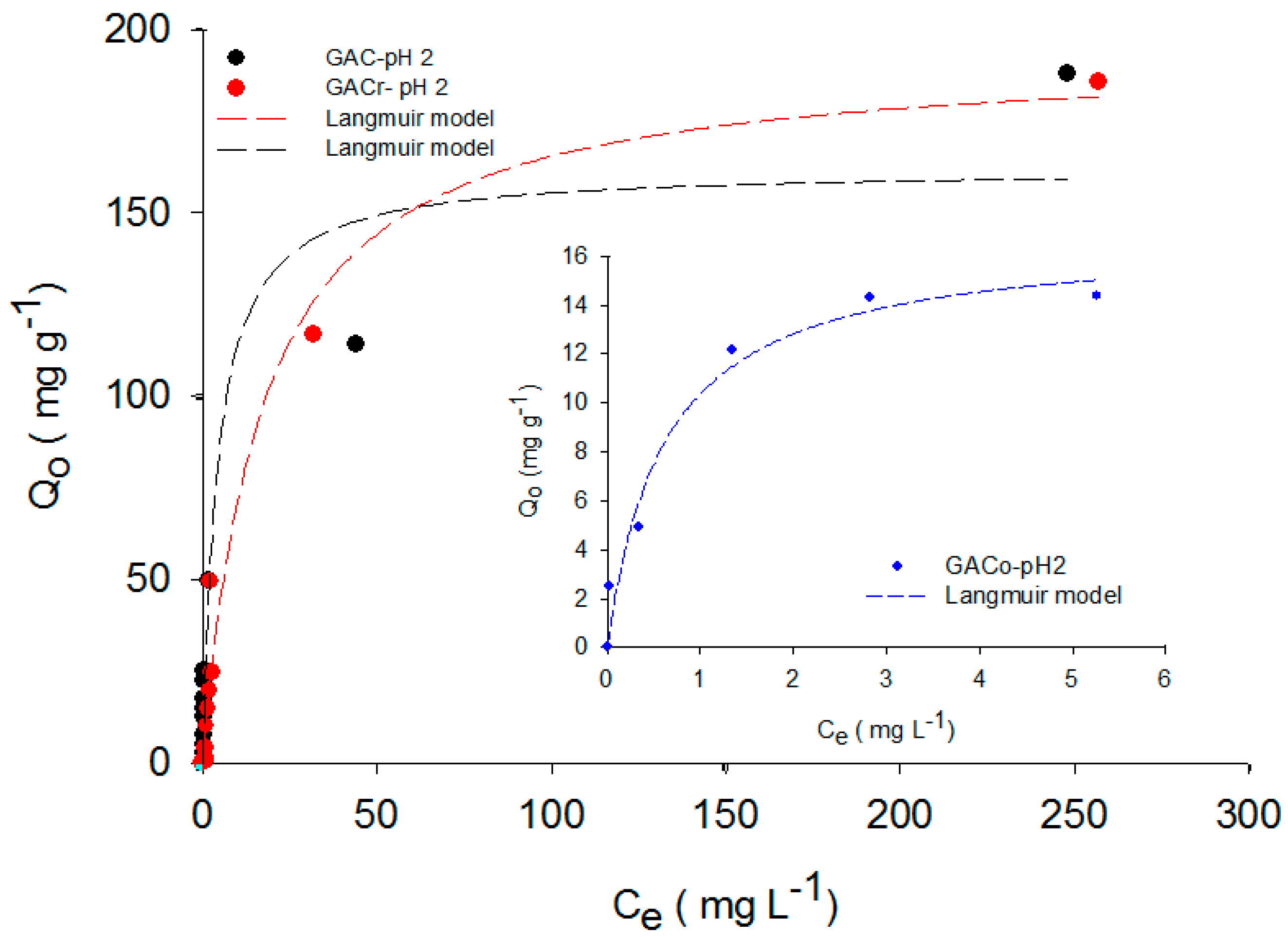
Figure 4 shows the paracetamol adsorption isotherms in acidic medium (pH 2) onto GAC, GACo and GACr.
Compared with the data at pH 7, a decrease in the adsorption capacity at lower pH for all the investigated adsorbents was observed, possibly due to the formation of paracetamol dimers in the solution and to the repulsions between the activated carbon surface having positive charges as well as the carbocation of paracetamol. GACo had the lowest performance, even when lower concentrations were used. In all cases, the activated carbon surface was polarized due to the hydronium ions present, favoring hydrogen bond formation with the solvent [
17,
19]. As adsorption capacity decreases with decreasing solution pH, it can be confirmed that the donor-acceptor electron mechanism is less active in this adsorption system. In order to complete the analysis of pH effect, in
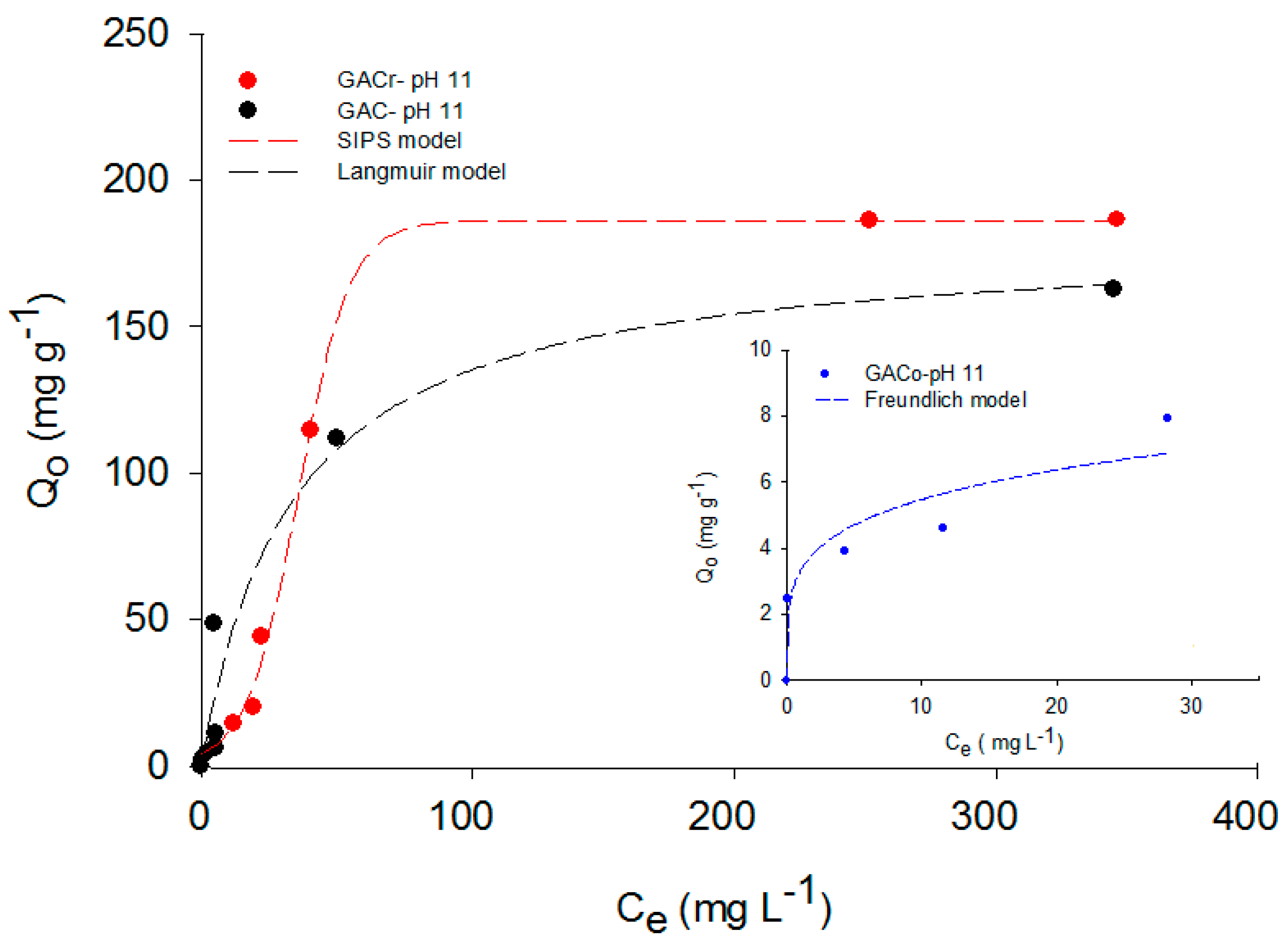
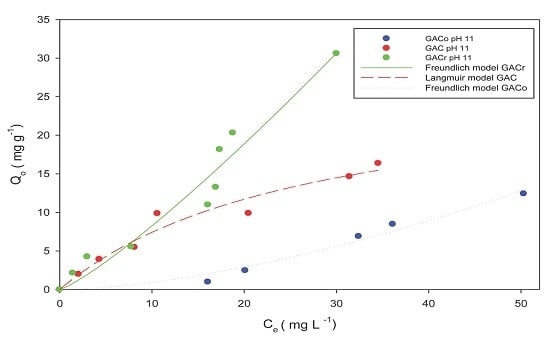
Figure 5, paracetamol adsorption isotherms at pH 11 are reported.
The ranking between adsorbents was maintained in this instance, and GACr showed the highest adsorption capacity. However, GACo and CACr showed a significant reduction in paracetamol adsorption capacity, when compared to data at neutral pH (
Figure 1). Indeed, at pH 11, paracetamol was present in its dissociated anionic form and the surface of all the activated carbons were negatively charged. Hence, repulsion phenomena are predominant and determine the observed reduction in adsorption capacity. On contrast, GAC had similar adsorption capacity at pH 11 compared to adsorption at neutral pH. However, the processes are described by different models, this may be due to a slight curvature at low concentrations of paracetamol at pH 11 due to the heterogeneity of the adsorbents accentuated by the presence of hydroxyl groups in the medium, which interact with the surface acid groups of the adsorbate. Differently, at paracetamol concentrations higher than 200 mg L
−1 the isotherms could be described with the same mathematical model.” Thus, paracetamol adsorption on GAC seems to be less dependent on specific interactions between functional groups on the activated carbon surface and the adsorbate.
The effect of pH on paracetamol adsorption has not been widely studied. Ferreira et al. [
18] studied paracetamol adsorption at pH values of 2, 6.5 and 11 on activated carbon prepared from coconut shell, whose properties are comparable to those of the GAC investigated in the present work. Similar to the result obtained in the present study, they determined that adsorption is highest at pH 6.5, at which a 75 mg g
−1 adsorption capacity was achieved in paracetamol solutions with an initial concentration of 50 mg L
−1, while at pH 11, the adsorption capacity decreased to 43 mg g
−1.
The results obtained in the present study were consistent with those reported Galhetas et al. [
19], which confirmed that paracetamol adsorption was favoured for reduced activated carbons and in neutral pH solutions.
In order to extend the analysis of the entire adsorption data set, paracetamol adsorption isotherms were adjusted to the Langmuir, Sips and Freundlich models. The modelling results are reported in
Figure 1,
Figure 4 and
Figure 5 for adsorption data at neutral, acid and basic pH values, respectively. For each adsorption isotherm, the best fitting model was reported.
The Langmuir model assumes that the maximum adsorption capacity corresponds to a monolayer of adsorbate molecule on the adsorbent surface. It is also assumed that adsorbate molecules bind to specific sites and each site accommodates one molecule. It is further assumed that the adsorptive energy is equal for all sites regardless of adsorbed molecules in neighboring sites, the adsorbent surface is flat, smooth and adsorbate-adsorbate interactions are negligible. Equation (1) describes the Langmuir model [
20,
21]:
where
qe represent the paracetamol adsorption quantity,
Qm is the maximum adsorption capacity and corresponds to the monolayer,
Ce is the paracetamol concentration in equilibrium and
KL is the Langmuir constant.
The Freundlich model is an empirical model, and it is frequently used to describe the adsorption of organic compounds in aqueous solution. It hypothesizes an exponential decay in the distribution of the adsorption energies of the active sites. Its mathematical representation is given in Equation (2) [
20,
21]:
where the constants
KF and
n depend on the interaction adsorbent-solute and on the temperature. The
n−1 values may be less or greater than unity, when the value is less than unity indicate a favourable adsorption.
The Sips model combines the Langmuir and Freundlich expressions to predict the adsorption in heterogeneous systems. Equation (3) shows the mathematical expression of this model [
21]:
where
Qm is the maximum adsorption capacity,
Ks and
ns are constants.
At low concentrations, Equation (3) is reduced to the Freundlich model, while at high concentrations; the maximum adsorption capacity corresponds to monolayer formation, as indicated by Langmuir model. The model parameters are directly related to the variations in the system properties, such as pH, concentration and temperature.
Table 3 shows the best fitting models and the relative parameters determined for paracetamol adsorption on the three activated carbons at different pH values.
The maximum adsorption capacity was determined by adjusting the data of the mentioned models and, as expected, paracetamol adsorption at pH 7 was the highest for all the activated carbons. For example, for GACo and GAC, the paracetamol adsorbed quantity at pH 2 and 11 was reduced to the tenth of the adsorbed amount at pH 7.
From a thermodynamic point of view, the adsorption process involves the loss of degrees of freedom due to the transition of adsorbate from three to two dimensional phase. This transition or loss in degrees of freedom quantitatively determined the values of the thermodynamic potentials, Gibbs energy, entropy and enthalpy.
In a heterogeneous system such as activated carbon, Gibbs energy decreases due to the reduction in the attractive force balance on the adsorbent surface. Thus, the process is spontaneous. By reducing the adsorbate’s degrees of freedom, the entropy change is negative. Therefore, from Equation (4) it is clear that the adsorption enthalpy change is negative [
22]:
The thermal effects of paracetamol adsorption on activated carbon can be determined by immersion calorimetry. This technique measures the energy changes associated with water molecule desorption from the activated carbon surface and subsequent paracetamol adsorption after contact with the activated carbon [
23].
Immersion enthalpy is produced by specific and non-specific active interactions during adsorption and is considered an important parameter to fully characterize solid-liquid systems. Its value depends on multiple factors such as surface area and adsorbent polarity [
24]. Equation (5) describes the immersion enthalpy in terms of the interactions occurring in the system.
where
represents the interactions asdorbate-activated carbon,
the interactions solvent-activated carbon and
the interactions solvent-solvent. The interactions adsorbate-adsorbate can be neglected with good approximation.
The interaction enthalpy corresponding to the energy produced by the contact between the adsorbate and the adsorbent, also neglecting the solvent-solvent interactions, is determined by Hess’s Law from the immersion enthalpy and activated carbon-solvent interactions:
The two values of immersion enthalpy show the differences in the interactions between the adsorbate and the activated carbon with different functional groups on the surface and, in association with Gibbs energy and entropy values, can supplement the information provided by adsorption isotherms [
24].
Table 4 shows the paracetamol immersion and interaction enthalpies on the three activated carbons at six different paracetamol concentrations (10, 50, 100, 200, 500 and 1000 mg L
−1).
In general, the immersion enthalpy increases (in absolute value) with this ranking GACo > GAC > GACr, confirming the influence of the carbon surface functionalities in the network of phenomena occurring in solution. The magnitude of interaction enthalpy follows an opposite trend, which can be ascribed to the different interactions of the solvent (water) with the activated carbons. The interaction once again confirmed the influence of surface functional groups. In fact, the ranking of water immersion enthalpy at neutral pH was GACo > GAC > GACr, and the values were −66.6, −49.7 and −32.4 J g−1. This result is due to the increased interaction with the solvent and the hydrogen bond formation between water and oxygen groups on the adsorbent surface. In basic medium, the enthalpy values follow the same trend as at neutral pH (−97.5, −57.3 and −35.1 J g−1 for GACo, GAC and GACr, respectively). These values reflect the acid-basic interactions that occur between the acidic functional groups on the activated carbons and the hydroxyls in the medium. Moreover, GACr has a notably lower value due to the absence of carboxylic functional groups. In acidic medium, the behavior of immersion enthalpy is opposite at pH 11. The GACr has an enthalpy value of −58.2 J g−1, followed by GAC (−51.4 J g−1), and finally GACo (−42.8 J g−1).
As an example, GACr in a paracetamol solution of 1000 mg L−1 at pH 7 presented an immersion enthalpy value of −36.0 J g−1. For the same system, the interaction enthalpy was 68.4 J g−1, confirming that paracetamol adsorption is favoured on basic activated carbons at neutral pH values, due to multiple factors, such as a: (a) decrease in the competitive adsorption with the solvent due to less hydrogen bond formation; (b) lower excess of protons that interact with π electrons on the graphenic layers and the aromatic ring of paracetamol, facilitating the donor-electron acceptor complex formation. The interaction enthalpies were positive, possibly because paracetamol adsorption is associated with the desorption of water or solvent from the activated carbon surfaces and this requires energy from the surroundings.
In order to give a deeper insight on adsorption mechanism, Gibbs energy change of the systems were also analyzed. Gibbs energy change of paracetamol adsorption on activated carbon provides information about the spontaneity of the process, as well as changes in the chemical potential of the system when there are variations in conditions, such as concentration and pH. The Dubinin-Radushkevich equation expresses the chemical potential for adsorption in the aqueous phase as [
25]:
where
Co/
Ce represents the ratio between the initial and equilibrium concentration,
R is the universal gas constant and
T is temperature in Kelvin. The chemical potential corresponds to molar Gibbs free energy of a component.
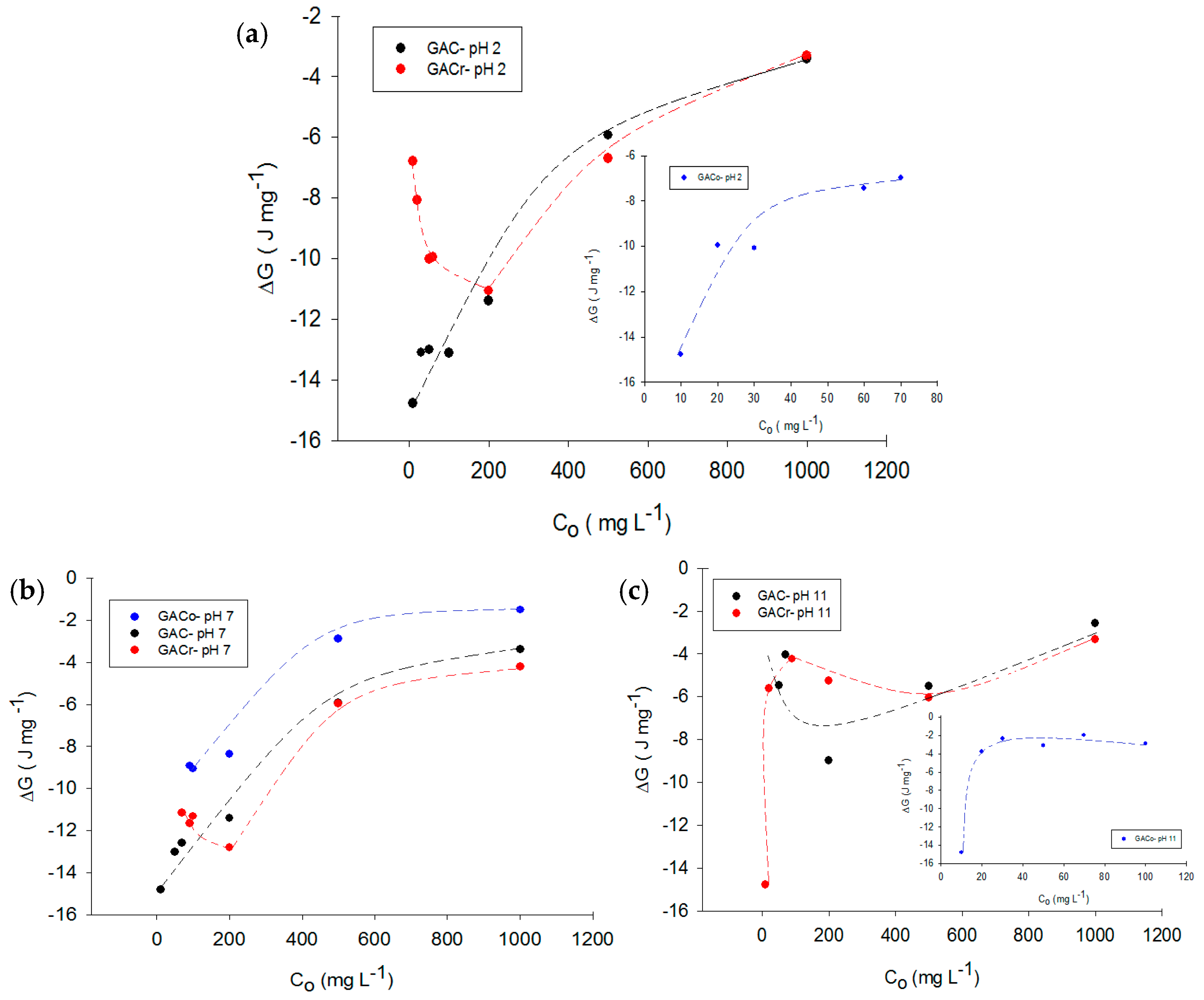
Figure 6a–c depict the Gibbs energy change for paracetamol adsorption on the three types of activated carbon at acidic, neutral and basic medium, respectively.
As expected, all Gibbs free energy changes were negative, as adsorption is spontaneous phenomenon.
Figure 6 shows the change in Gibbs energy. For GACr, adsorption is favoured at neutral pH, as under these conditions adsorbate-adsorbent interactions increase the enthalpic component, which is directly related to Gibbs energy change (see Equation (7)).
The potential for GACr, at pH 2 and 7 decreases at low paracetamol concentrations and then increases with increased paracetamol concentration. This trend is due to the presence of few adsorbate molecules in solution at low concentrations, which leads to the desorption of solvent molecules from the surface of the adsorbent. Paracetamol adsorption requires less energy from the surroundings. When the paracetamol concentration increases the energy also increases changes due to the formation of adsorbate-adsorbent interactions. At pH 11, Gibbs energy change increases almost monotonically in all systems. For GACo, Gibbs energy change are qualitatively independent of the pH, as an increase in the equilibrium concentration determines a decrease in Gibbs energy, due to an increase in interactions between functional groups on the activated carbon and the adsorbate. A decrease in pH is related to an increase in the Gibbs energy change because at acid pH a greater amount of hydronium ions in the medium are adsorbed by activated carbon, decreasing the adsorption and interaction capacity of paracetamol.
Finally, for GAC, no single trend for change of Gibbs energy with pH was found, due to the amphoteric characteristics of the surface. At pH extreme values of 2 and 11, the change in Gibbs energy was values above the calculated energy change for the process at pH 7 under the same equilibrium concentration. This indicates that pH change did not favour the adsorption process in this activated carbon.