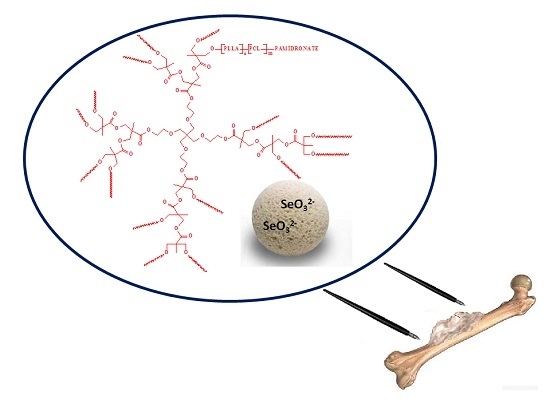
Pamidronate-Conjugated Biodegradable Branched Copolyester Carriers: Synthesis and Characterization
Abstract
:1. Introduction
2. Results
2.1. Copolymeric Branched Matrices Synthesis and Characterization
2.2. In vitro PAM Release Characteristic and the Branched Copolymer Conjugates Synthesis
3. Materials and Methods
3.1. General Information
3.2. Synthesis of the Branched Copolymeric Carriers
3.3. Spectral Characterization of Copolymeric Biodegradable Matrices (bis-MPA-PLLA90-PKL38 (1H-NMR and FT-IR) and bis-MPA-PLLA38-PCL90 (13C-NMR) as Examples)
3.4. Synthesis of the bis-MPA-PLLA50-PCL50-PAM, bis-MPA-PLLA90-PCL38-PAM and bis-MPA-PLLA38-PCL90-PAM Branched Conjugates
3.5. Toxicity Assays
3.5.1. Microtox Assay
3.5.2. Spirotox Test
3.5.3. The Umu-Test
3.6. Fabrication of the Hydroxyapatite Granules Doped with Selenite Ion
3.7. Fabrication of the Porous Material—bis-MPA-PLLA-PCL-PAM-hydroxyapatite Doped with Selenium Ion for In Vitro and Hydrolytic Biodegradation Studies
3.8. In Vitro PAM Release Studies form the Synthesized Conjugates Coated Hydroxyapatite Porous Granules Doped with Selenite Ions
3.9. Hydrolytic Degradation of the Synthesized Copolymeric Matrices Coated Hydroxyapatite Porous Granules Doped with Selenite Ion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Larsona, N.; Ghandeharia, H. Polymeric conjugates for drug delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Nevozhay, D.; Kańska, U.; Budzyńska, R.; Boratyński, J. Contemporary state of research on conjugates and other drug delivery systems in the treatment of cancer and other diseases. Postep. Hig. Med. Dosw. 2007, 61, 350–360. [Google Scholar]
- Fitton, A.; McTavish, D. Pamidronate. A review of its pharmacological properties and therapeutic efficacy in resorptive bone disease. Drugs 1992, 43, 145–318. [Google Scholar] [CrossRef]
- Russell, R.; Graham, G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Sarpietro, M.G.; Castelli, F. Synthesis and biological evaluation of a new polymeric conjugate and nanocarrier with osteotropic properties. J. Funct. Biomater. 2013, 3, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Takashima, M.; Sano, J.-I.; Nishiyama, K. Development of polyethylene glycol-conjugated alendronate, a novel nitrogen-containing bisphosphonate derivative: Evaluation of absorption, safety, and effects afterintrapulmonary administration in rats. J. Pharm. Sci. 2011, 100, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, G.; Chi, H.; Wang, D. Alendronate-conjugated amphiphilic hyperbranched polymer based on Boltorn H40 and poly(ethylene glycol) for bone-targeted drug delivery. Bioconj. Chem. 2012, 23, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jo, J.; Kawai, Y.; Aoki, I. Preparation of polymer-based multimodal imaging agent to visualize the process of bone regeneration. J. Control Release 2012, 157, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Cattalini, J.P.; Boccaccini, A.R.; Lucangioli, S.; Mourino, V. Bisphosphonate-based strategies for bone tissue engineering and orthopedic implants. Tissue Eng. Part B Rev. 2012, 18, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Mourino, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Broström, J.; Boss, A.; Chronakis, I.S. Biodegradable films of partly branched poly(l-lactide)-co-poly(epsilon-caprolactone) copolymer: Modulation of phase morphology, plasticization properties and thermal depolymerization. Biomacromolecules 2004, 5, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and evaluation of a star amphiphilic block copolymer from poly(ε-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconj. Chem. 2005, 16, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Sobczak, M.; Olędzka, E.; Nałęcz-Jawecki, G.; Dębek, C. Synthesis, Characterization and in Vitro Evaluation of New Composite Bisphosphonate Delivery Systems. Int. J. Mol. Sci. 2014, 15, 16831–16847. [Google Scholar] [CrossRef] [PubMed]
- Fabianowski, W.; Polak, B.; Lewandowska-Szumieł, M. Polymers for bone reconstruction—Evaluation of chosen polymeric substrates in osteoblast in vitro culture. Polimery 2004, 49, 522–529. [Google Scholar]
- Ashby, M. Selection of Materials in Engineering Design; Wydawnictwo Naukowo-Techniczne: Warszawa, Poland, 1998; pp. 25–40. [Google Scholar]
- Błażewicz, S.; Stoch, L. Polymeric biomaterials. In Biomaterials, 1st ed.; Nałęcz, M., Ed.; Akademicka Oficyna Wydawnicza Exit: Warsaw, Poland, 2003; Volume 4, pp. 85–120. [Google Scholar]
- Fitak, E.; Wagner, L. The role of selenium role—Importance in medicine and dentistry based on the literature. Nowa Stomatol. 2009, 3, 82–84. [Google Scholar]
- Kolmas, J.; Oledzka, E.; Sobczak, M.; Nałęcz-Jawecki, G. Nanocrystalline hydroxyapatite doped with selenium oxyanions: A new material for potential biomedical applications. Mat. Sci. Eng. C Mater. 2014, 39, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Seshima, H.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Inoue, T.; Oda, Y. Control of bisphosphonate release using hydroxyapatite granules. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Oledzka, E.; Kaliszewska, D.; Sobczak, M.; Raczak, A.; Nickel, P.; Kolodziejski, W. Synthesis and properties of a star-shaped poly(ε-caprolactone)-ibuprofen conjugate. J. Biomater. Sci. Polym. Ed. 2012, 23, 2039–2054. [Google Scholar] [PubMed]
- Sobczak, M.; Debek, C.; Oledzka, E.; Nalecz-Jawecki, G.; Kolodziejski, W.L.; Rajkiewicz, M. Segmented polyurethane elastomers derived from aliphatic polycarbonate and poly(ester-carbonate) soft segments for biomedical applications. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3904–3913. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Ndwabu, S. Evaluation of whey protein isolate-graft-carbopolpolyacrylamide pH-sensitive composites for controlled release of pamidronate. Polym. Bull. 2017. [Google Scholar] [CrossRef]
- Weidenauer, U.; Bodmer, D.; Kissel, T. Microencapsulation of hydrophilic drug substances using biodegradable polyesters. Part II: Implants allowing controlled drug release—A feasibility study using bisphosphonates. J. Microencapsul. 2004, 21, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Nalecz-Jawecki, G. Spirotox test—Spirostomum ambiguum acute toxicity test. In Small-Scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.-F., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 299–322. [Google Scholar]
- International Standard ISO/FDIS 13829. Water Quality—Determination of the Genotoxicity of Water and Waste Water Using the Umu-Test. 2000. Available online: https://www.iso.org/standard/22626.html (accessed on 22 June 2017).
Sample Availability: Samples of the compounds are available from the authors. |





| Entry | [I]/[LLA]/[CL] | Yield (%) | DP a | DS a | Mn(SEC-MALLS) b | Mw/Mn b |
|---|---|---|---|---|---|---|
| bis-MPA-PLLA64-PCL64 | 1/64/64 | 82 | 25 | 13 | 21000 | 1.5 |
| bis-MPA-PLLA90-PCL38 | 1/90/38 | 85 | 22 | 10 | 18800 | 1.6 |
| bis-MPA-PLLA38-PCL90 | 1/38/90 | 97 | 19 | 11 | 19500 | 1.5 |
| Entry | Microtox 15 min-PE 1 0.9 mg mL−1 | Microtox 30 min-PE 1 0.9 mg mL−1 | Spirotox 24 h-PE 1 |
|---|---|---|---|
| bis-MPA-PLLA64-PCL64 | 10 ± 1 | 11 ± 2 | 0 |
| bis-MPA-PLLA90-PCL38 | 14 ± 2 | 18 ± 1 | 0 |
| bis-MPA-PLLA38-PCL90 | 4 ± 2 | 5 ± 1 | 0 |
| Entry | −S9 | +S9 | ||
|---|---|---|---|---|
| G ± SD | IR ± SD | G ± SD | IR ± SD | |
| bis-MPA-PLLA64-PCL64 | 1.09 ± 0.03 | 0.81 ± 0.09 | 1.18 ± 0.04 | 0.89 ± 0.09 |
| bis-MPA-PLLA90-PCL38 | 1.06 ± 0.05 | 0.88 ± 0.07 | 1.16 ± 0.02 | 0.95 ± 0.03 |
| bis-MPA-PLLA38-PCL90 | 0.98 ± 0.05 | 0.96 ± 0.02 | 1.15 ± 0.03 | 0.96 ± 0.04 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oledzka, E.; Pachowska, D.; Orłowska, K.; Kolmas, J.; Drobniewska, A.; Figat, R.; Sobczak, M. Pamidronate-Conjugated Biodegradable Branched Copolyester Carriers: Synthesis and Characterization. Molecules 2017, 22, 1063. https://doi.org/10.3390/molecules22071063
Oledzka E, Pachowska D, Orłowska K, Kolmas J, Drobniewska A, Figat R, Sobczak M. Pamidronate-Conjugated Biodegradable Branched Copolyester Carriers: Synthesis and Characterization. Molecules. 2017; 22(7):1063. https://doi.org/10.3390/molecules22071063
Chicago/Turabian StyleOledzka, Ewa, Dagmara Pachowska, Katarzyna Orłowska, Joanna Kolmas, Agata Drobniewska, Ramona Figat, and Marcin Sobczak. 2017. "Pamidronate-Conjugated Biodegradable Branched Copolyester Carriers: Synthesis and Characterization" Molecules 22, no. 7: 1063. https://doi.org/10.3390/molecules22071063
APA StyleOledzka, E., Pachowska, D., Orłowska, K., Kolmas, J., Drobniewska, A., Figat, R., & Sobczak, M. (2017). Pamidronate-Conjugated Biodegradable Branched Copolyester Carriers: Synthesis and Characterization. Molecules, 22(7), 1063. https://doi.org/10.3390/molecules22071063