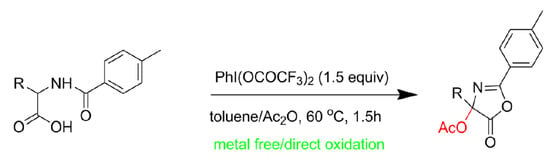
A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine
Abstract
Share and Cite
Wen, G.; Zhang, W.-X.; Wu, S. A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine. Molecules 2017, 22, 1102. https://doi.org/10.3390/molecules22071102
Wen G, Zhang W-X, Wu S. A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine. Molecules. 2017; 22(7):1102. https://doi.org/10.3390/molecules22071102
Chicago/Turabian StyleWen, Gang, Wen-Xuan Zhang, and Song Wu. 2017. "A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine" Molecules 22, no. 7: 1102. https://doi.org/10.3390/molecules22071102
APA StyleWen, G., Zhang, W.-X., & Wu, S. (2017). A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine. Molecules, 22(7), 1102. https://doi.org/10.3390/molecules22071102