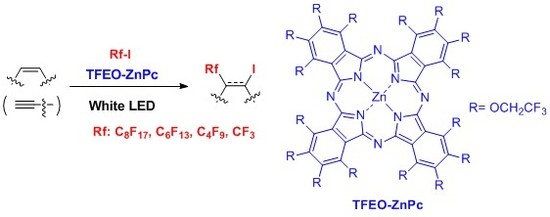
Trifluoroethoxy-Coated Phthalocyanine Catalyzes Perfluoroalkylation of Alkenes under Visible-Light Irradiation †
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Perfluoroalkylation of Alkenes and Alkynes with TFEOZnPc
3.1.1. 5-Iodo-6-perfluorooctylhexane-1-ol (2aa)
3.1.2. 5-Iodo-6-perfluorohexylhexanol (2ab)
3.1.3. 5-Iodo-6-perfluorobutylhexanol (2ac)
3.1.4. 5-Iodo-6-trifluoromethylhexanol (2ad)
3.1.5. 5-Iodo-6-perfluorooctylhexyll-4-methylbenzenesulfonate (2b)
3.1.6. 1-Bromo-5-iodo-6-perfluorooctylhexane (2c)
3.1.7. 1,5-Diiodo-6-perfluorooctylhexane (2d)
3.1.8. tert-Butyl (2-iodo-3-perfluorooctylpropyl)carbamate (2e)
3.1.9. (3-Iodo-4-perfluorooctylbutyl)benzene (2f)
3.1.10. 3-Iodo-4-perfluorooctyldobut-3-en-1-ol (2g)
3.1.11. 2-Iodo-1-perfluorooctyloctene (2h)
3.1.12. 5-Iodo-6-perfluorooctylhexane-2-one (2i)
3.1.13. 1-Iodo-2-perfluorooctylcyclohexane (2j)
3.1.14. 2-Iodo-3-perfluorooctylnorbornane (2k)
3.1.15. 3-Iodo-4-(perfluorooctyl)butanenitrile (2l)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Ma, J.A.; Cahard, D. Update 1 of: Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev. 2008, 108, PR1–PR43. [Google Scholar] [CrossRef] [PubMed]
- Barata-Vallejo, S.; Bonesi, S.M.; Postigo, A. Perfluoroalkylation reactions of (hetero)arenes. RSC Adv. 2015, 5, 62498–62518. [Google Scholar] [CrossRef]
- Besset, T.; Poisson, T.; Pannecoucke, X. 1,4-Addition of the CF3 Group, perfluoroalkyl groups and functionalized difluoromethylated moieties: An overview. J. Fluor. Chem. 2015, 178, 225–240. [Google Scholar] [CrossRef]
- Sugiishi, T.; Amii, H.; Aikawa, K.; Mikami, K. Carbon–carbon bond cleavage for Cu-mediated aromatic trifluoromethylations and pentafluoroethylations. Beilstein J. Org. Chem. 2015, 11, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Hu, J. The unique fluorine effects in organic reactions: Recent facts and insights into fluoroalkylations. Chem. Soc. Rev. 2016, 45, 5441–5454. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T.; Jahan, I.; Tonoi, T.; Shinmen, M.; Nishikawa, A.; Yamaguchi, K.; Sekine, I.; Nagano, H. Photoinduced addition and addition-elimination reactions of perfluoroalkyl iodides to electron-deficient olefins. Tetrahedron 2012, 68, 6856–6861. [Google Scholar] [CrossRef]
- Tsuchii, K.; Imura, M.; Kamada, N.; Hirao, T.; Ogawa, A. An Efficient Photoinduced Iodoperfluoroalkylation of Carbon-Carbon Unsaturated Compounds with Perfluoroalkyl Iodides. J. Org. Chem. 2004, 69, 6658–6665. [Google Scholar] [CrossRef] [PubMed]
- Nogami, E.; Washimi, Y.; Yamazaki, T.; Kubota, T.; Yajima, T. Photoinduced double perfluoroalkylation of methylacenes. Tetrahedron Lett. 2016, 57, 2624–2627. [Google Scholar] [CrossRef]
- Haszeldin, R.N. The reactions of fluorocarbon radicals. Part I. The reaction of iodotrifluoromethane with ethylene and tetrafluoroethylene. J. Chem. Soc. 1949, 2856–2861. [Google Scholar] [CrossRef]
- Tarrant, P.; Stump, E.C., Jr. Free-Radical Additions Involving Fluorine Compounds. VII. The Addition of Perhaloalkanes to Vinyl Ethyl Ether and Vinyl 2,2,2-Trifluoroethyl Ether. J. Org. Chem. 1964, 29, 1198–1202. [Google Scholar] [CrossRef]
- König, B. Chemical Photocatalysis; Walter de Gruyter: Berlin, Germany, 2013; p. 139. ISBN 978-3-11-026924-6. [Google Scholar]
- Albini, A. Photochemistry: Past, Present and Future; Springer: Berlin, Germany, 2015; pp. 95–97. ISBN 978-3-662-47977-3. [Google Scholar]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Peña-López, M.; Rosas-Hernández, A.; Beller, M. Progress on All Ends for Carbon–Carbon Bond Formation through Photoredox Catalysis. Angew. Chem. Int. Ed. 2015, 54, 5006–5008. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.K.; Lang, S.B.; Heitz, D.R.; Molander, G.A. Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development. ACS Catal. 2017, 7, 2563–2575. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.D.; Tucker, J.W.; Konieczynska, M.D.; Stephenson, C.R.J. Intermolecular Atom Transfer Radical Addition to Olefins Mediated by Oxidative Quenching of Photoredox Catalysts. J. Am. Chem. Soc. 2011, 133, 4160–4163. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Choi, S.; Kim, E.; Cho, E.J. Trifluoromethylation of Alkenes by Visible Light Photoredox Catalysis. J. Org. Chem. 2012, 77, 11383–11387. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, C.J.; Nguyen, J.D.; Finkbeiner, P.; Stephenson, C.R.J. Visible Light-Mediated Atom Transfer Radical Addition via Oxidative and Reductive Quenching of Photocatalysts. J. Am. Chem. Soc. 2012, 134, 8875–8884. [Google Scholar] [CrossRef] [PubMed]
- Tomita, R.; Yasu, Y.; Koike, T.; Akita, M. Combining Photoredox-Catalyzed Trifluoromethylation and Oxidation with DMSO: Facile Synthesis of α-Trifluoromethylated Ketones from Aromatic Alkenes. Angew. Chem. Int. Ed. 2014, 53, 7144–7148. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Jung, J.; Park, S.; Cho, E.J. Controlled Trifluoromethylation Reactions of Alkynes through Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2014, 53, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Jarrige, L.; Carboni, A.; Dagousset, G.; Levitre, G.; Magnier, E.; Masson, G. Photoredox-Catalyzed Three-Component Tandem Process: An Assembly of Complex Trifluoromethylated Phthalans and Isoindolines. Org. Lett. 2016, 18, 2906–2909. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Iqbal, N.; You, Y.; Cho, E.J. Controlled Fluoroalkylation Reactions by Visible-Light Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 2284–2294. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yuan, L.; Li, Z.Z.; Peng, Z.Y.; Deng, Y.L.; Wang, L.N.; Huang, G.X.; Sheng, R.L. Visible-light-induced dearomative spirocyclization of N-benzylacrylamides toward perfluorinated azaspirocyclic cyclohexadienones. Tetrahedron Lett. 2017, 58, 2127–2130. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, T.; Chen, F.; Mehrkens, N.; Rominger, F.; Rudolph, M.; Hashmi, A.S.K. Gold-Catalyzed Highly Selective Photoredox C(sp2)–H Difluoroalkylation and Perfluoroalkylation of Hydrazones. Angew. Chem. Int. Ed. 2016, 55, 2934–2938. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yu, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Monofluoroalkenylation of Dimethylamino Compounds through Radical-Radical Cross-Coupling. Angew. Chem. Int. Ed. 2016, 55, 9416–9421. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Photoredox-Controlled Mono- and Di-Multifluoroarylation of C(sp3)–H Bonds with Aryl Fluorides. Angew. Chem. Int. Ed. 2017, 56, 7266–7270. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, J.; Wurm, T.; Weingand, V.; Sung, H.L.; Rominger, F.; Rudolph, M.; Hashmi, A.S.K. A general photoinduced electron transfer-directed chemoselective perfluoroalkylation of N,N-dialkylhydrazones. Org. Chem. Front. 2016, 3, 841–845. [Google Scholar] [CrossRef]
- Neumann, M.; Füldner, S.; König, B.; Zeitler, K. Metal-Free, Cooperative Asymmetric Organophotoredox Catalysis with Visible Light. Angew. Chem. Int. Ed. 2011, 50, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Pitre, S.P.; McTiernan, C.D.; Ismaili, H.; Scaiano, J.C. Metal-Free Photocatalytic Radical Trifluoromethylation Utilizing Methylene Blue and Visible Light Irradiation. ACS Catal. 2014, 4, 2530–2535. [Google Scholar] [CrossRef]
- Yajima, T.; Ikegami, M. Metal-Free Visible-Light Radical Iodoperfluoroalkylation of Terminal Alkenes and Alkynes. Eur. J. Org. Chem. 2017, 2126–2129. [Google Scholar] [CrossRef]
- McKeown, N.B. Phthalocyanine Materials: Synthesis; Structure and Function; Cambridge University Press: Cambridge, UK, 1998; pp. 2–4. ISBN 0521496233. [Google Scholar]
- Bekaroğlu, Ö. Functional Phthalocyanine Molecular Materials; Springer Science and Business Media: Berlin, Germany, 2010; pp. 1–3. ISBN 978-3-642-04752-7. [Google Scholar]
- Mack, J.; Kobayashi, N. Low Symmetry Phthalocyanines and Their Analogues. Chem. Rev. 2011, 111, 281–321. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gomis, L.; Fernández-Lázaro, F.; Sastre-Santos, Á. Advances in phthalocyanine-sensitized solar cells (PcSSCs). J. Mater. Chem. A 2014, 2, 15672–15682. [Google Scholar] [CrossRef]
- Ragoussi, M.E.; Mine, I.; Torres, T. Recent Advances in Phthalocyanine-Based Sensitizers for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2013, 6475–6489. [Google Scholar] [CrossRef]
- Gerdes, R.; Lapok, L.; Tsaryova, O.; Wöhrle, D.; Gorun, S.M. Rational design of a reactive yet stable organic-based photocatalyst. Dalton Trans. 2009, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.B. Phthalocyanine Metal Complexes in Catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.R.; Shibata, N.; Kondo, Y.; Nakamura, S.; Toru, T. Design, Synthesis, and Spectroscopic Investigation of Zinc Dodecakis (trifluoroethoxy) phthalocyanines Conjugated with Deoxyribonucleosides. Angew. Chem. Int. Ed. 2006, 45, 8163–8166. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Umeda, M.; Tokunaga, E.; Toru, T.; Shibata, N. Synthesis of Benzene-centered Trinuclear Phthalocyanines by Triple-click Chemistry. Chem. Lett. 2010, 39, 337–339. [Google Scholar] [CrossRef]
- Yamada, I.; Umeda, M.; Hayashi, Y.; Soga, T.; Shibata, N. Fundamental Study on Organic Solar Cells Based on Soluble Zinc Phthalocyanine. Jpn. J. Appl. Phys. 2012, 51, 04DK09-1–04DK09-6. [Google Scholar] [CrossRef]
- Shibata, N.; Mori, S.; Hayashi, M.; Umeda, M.; Tokunaga, E.; Shiro, M.; Sato, H.; Hoshi, T.; Kobayashi, N. A phthalocyanine–subphthalocyanine heterodinuclear dimer: Comparison of spectroscopic properties with those of homodinuclear dimers of constituting units. Chem. Commun. 2014, 50, 3040–3043. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Ogawa, N.; Tokunaga, E.; Shibata, N. Synthesis and optical properties of trifluoroethoxysubstituted double-decker phthalocyanines. J. Porphyr. Phthalocyanines 2014, 18, 1034–1041. [Google Scholar] [CrossRef]
- Obata, T.; Mori, S.; Suzuki, Y.; Kashiwagi, T.; Tokunaga, E.; Shibata, N.; Tanaka, M. Photodynamic Therapy Using Novel Zinc Phthalocyanine Derivatives and a Diode Laser for Superficial Tumors in Experimental Animals. J. Cancer Ther. 2015, 6, 53–61. [Google Scholar] [CrossRef]
- Satoru, M.; Norio, S. Development of Trifluoroethoxy Substituted Phthalocyanines and Subphthalocyanines and their Applications. J. Synth. Org. Chem. Jpn. 2016, 74, 154–166. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Hiromura, T.; Tokunaga, E.; Shibata, N. Trifluoroethoxy-Coated Subphthalocyanine affects Trifluoromethylation of Alkenes and Alkynes even under Low-Energy Red-Light Irradiation. ChemistryOpen 2017, 6, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Morse, G.E.; Bender, T.P. Boron Subphthalocyanines as Organic Electronic Materials. ACS Appl. Mater. Interfaces 2012, 4, 5055–5068. [Google Scholar] [CrossRef] [PubMed]
- Claessens, C.G.; González-Rodríguez, D.; Rodríguez-Morgade, M.S.; Medina, A.; Torres, T. Subphthalocyanines, Subporphyrazines, and Subporphyrins: Singular Nonplanar Aromatic Systems. Chem. Rev. 2014, 114, 2192–2277. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Kobayashi, N. Structurally-modified subphthalocyanines: Molecular design towards realization of expected properties from the electronic structure and structural features of subphthalocyanine. Chem. Commun. 2014, 50, 6949–6966. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Resnick, P.; Efimenko, K.; Genzer, J.; DeSimone, J.M. Alternative Fluoropolymers to Avoid the Challenges Associated with Perfluorooctanoic Acid. Ind. Eng. Chem. Res. 2008, 47, 502–508. [Google Scholar] [CrossRef]
- Hang, Z.; Li, Z.; Liu, Z.Q. Iodotrifluoromethylation of Alkenes and Alkynes with Sodium Trifluoromethanesulfinate and Iodine Pentoxide. Org. Lett. 2014, 16, 3648–3651. [Google Scholar] [CrossRef] [PubMed]
- Beniazza, R.; Atkinson, R.; Absalon, C.; Castet, F.; Denisov, S.A.; McClenaghan, N.D.; Lastécouères, D.; Vincent, J.M. Benzophenone vs. Copper/Benzophenone in Light-Promoted Atom Transfer Radical Additions (ATRAs): Highly Effective Iodoperfluoroalkylation of Alkenes/Alkynes and Mechanistic Studies. Adv. Synth. Catal. 2016, 358, 2949–2961. [Google Scholar] [CrossRef]
- Lumbierres, M.; Moreno-Mañas, M.; Vallribera, A. Addition of perfluorooctyl iodide to alkenes. Catalysis by triphenylphosphane. Tetrahedron 2002, 58, 4061–4065. [Google Scholar] [CrossRef]
- When we attempted the reaction of 1a to 2aa under the best conditions and in the presence of NaN3 (3 equiv), the reaction was completely inhibited and the starting material was recovered. Further reactions will be investigated in the presence of a variety of nucleophiles
Sample Availability: Samples of the compounds TFEO-ZnPc and TFEO-SubPc are available from the authors. |




| Entry | Catalyst (1 mol %) | Additive (0.35 equiv) | Solvent | Yield (%) b |
|---|---|---|---|---|
| 1 | TFEO-ZnPc | Na ascorbate | MeCN/MeOH | 88 |
| 2 c | TFEO-ZnPc | Na ascorbate | MeCN/MeOH | <5 |
| 3 | - | Na ascorbate | MeCN/MeOH | <5 |
| 4 | TFEO-ZnPc | - | MeCN/MeOH | <5 |
| 5 | tBu-ZnPc | Na ascorbate | MeCN/MeOH | 45 |
| 6 | TFEO-SubPc | Na ascorbate | MeCN/MeOH | 77 |
| 7 | TFEO-ZnPc | Ascorbic acid | MeCN/MeOH | 33 |
| 8 | TFEO-ZnPc | Hantzsch ester | MeCN/MeOH | 24 |
| 9 d,e | TFEO-ZnPc | Na ascorbate | MeCN | <5 |
| 10 d | TFEO-ZnPc | Na ascorbate | MeOH | 62 |
| 11 f | TFEO-ZnPc | Na ascorbate | DMSO | 7 |
| 12 g | TFEO-ZnPc | Na ascorbate | MeCN/MeOH | 93 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaki, K.; Hiromura, T.; Amii, H.; Shibata, N. Trifluoroethoxy-Coated Phthalocyanine Catalyzes Perfluoroalkylation of Alkenes under Visible-Light Irradiation. Molecules 2017, 22, 1130. https://doi.org/10.3390/molecules22071130
Matsuzaki K, Hiromura T, Amii H, Shibata N. Trifluoroethoxy-Coated Phthalocyanine Catalyzes Perfluoroalkylation of Alkenes under Visible-Light Irradiation. Molecules. 2017; 22(7):1130. https://doi.org/10.3390/molecules22071130
Chicago/Turabian StyleMatsuzaki, Kohei, Tomoya Hiromura, Hideki Amii, and Norio Shibata. 2017. "Trifluoroethoxy-Coated Phthalocyanine Catalyzes Perfluoroalkylation of Alkenes under Visible-Light Irradiation" Molecules 22, no. 7: 1130. https://doi.org/10.3390/molecules22071130
APA StyleMatsuzaki, K., Hiromura, T., Amii, H., & Shibata, N. (2017). Trifluoroethoxy-Coated Phthalocyanine Catalyzes Perfluoroalkylation of Alkenes under Visible-Light Irradiation. Molecules, 22(7), 1130. https://doi.org/10.3390/molecules22071130