Design, Synthesis, and Biological Activity of New Triazole and Nitro-Triazole Derivatives as Antifungal Agents
Abstract
:1. Introduction
2. Results and Discussion
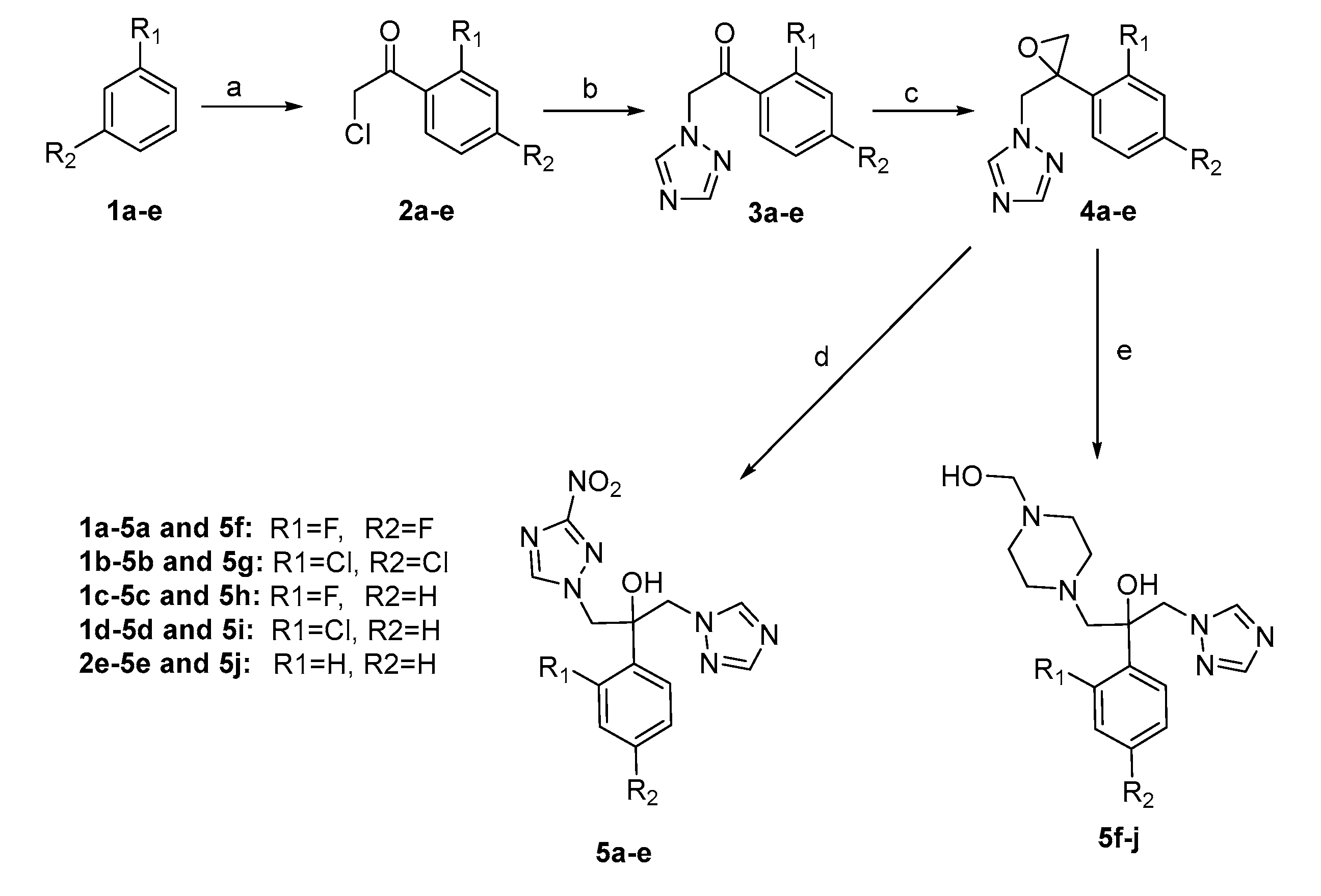
2.1. Chemistry
2.2. Biological Activity
2.3. Docking
2.4. Structure Activity Relationship
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of 2-Chloro-1-(2,4-disubstituted phenyl) Ethanone (2a–e)
3.1.2. General Procedure for the Synthesis of 1-(2,4-Disubstituted phenyl)-2-(1H-1,2,4-triazol-1-yl) Ethanone (3a–e)
3.1.3. General Procedure for the Synthesis of 1-((2-(2,4-Disubstituted phenyl)oxiran-2-yl)methyl)-1H-1,2,4-triazole (4a–e)
3.1.4. General Procedure for the Synthesis of 2-(2,4-Disubstituted phenyl)-1-(3-nitro-1H-1,2,4-triazol -1-yl)-3-(1H-1,2,4-triazol-1-yl)propan-2-ol (5a–e, series A)
3.1.5. General Procedure for the Synthesis of 2-(2,4-Disubstituted phenyl)-1-(3-nitro-1H-1,2,4-triazol-1-yl)-3-(1H-1,2,4-triazol-1-yl)propan-2-ol (5f–j, series B)
3.2. Docking Study
3.3. Biological Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spitzer, M.; Griffiths, E.; Blakely, K.M.; Wildenhain, J.; Ejim, L.; Rossi, L.; De Pascale, G.; Curak, J.; Brown, E.; Tyers, M. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 2011, 7, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A neglected epidemic: Fungal infections in HIV/AIDS. Trends Microbiol. 2014, 22, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Salari, S.; Khosravi, A.; Mousavi, S.; Nikbakht-Brojeni, G. Mechanisms of resistance to fluconazole in candida albicans clinical isolates from iranian hiv-infected patients with oropharyngeal candidiasis. J. Méd. Mycol. 2016, 26, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Batzlaff, C.M.; Limper, A.H. When to consider the possibility of a fungal infection: An overview of clinical diagnosis and laboratory approaches. Clin. Chest Med. 2017. [CrossRef]
- Lamb, D.C.; Kelly, D.E.; Venkateswarlu, K.; Manning, N.J.; Bligh, H.F.J.; Schunck, W.H.; Kelly, S.L. Generation of a complete, soluble and catalytically active sterol 14α-demethylase-reductase complex. Biochemistry 1999, 38, 8733–8738. [Google Scholar] [CrossRef] [PubMed]
- Bartroli, J.; Turmo, E.; Algueró, M.; Boncompte, E.; Vericat, M.L.; Conte, L.; Ramis, J.; Merlos, M.; García-Rafanell, J.; Forn, J. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J. Med. Chem. 1998, 41, 1869–1882. [Google Scholar] [PubMed]
- Upadhayaya, R.S.; Jain, S.; Sinha, N.; Kishore, N.; Chandra, R.; Arora, S.K. Synthesis of novel substituted tetrazoles having antifungal activity. Eur. J. Med. Chem. 2004, 39, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Lebouvier, N.; Giraud, F.; Corbin, T.; Na, Y.M.; Le Baut, G.; Marchand, P.; Le Borgne, M. Efficient microwave-assisted synthesis of 1-(1H-indol-1-yl)-2-phenyl-3-(1H-1,2,4-triazol-1-yl)propan-2-ols as antifungal agents. Tetrahedron Lett. 2006, 47, 6479–6483. [Google Scholar] [CrossRef]
- Lebouvier, N.; Pagniez, F.; Duflos, M.; Le Pape, P.; Na, Y.M.; Le Baut, G.; Le Borgne, M. Synthesis and antifungal activities of new fluconazole analogues with azaheterocycle moiety. Bioorg. Med. Chem. Lett. 2007, 17, 3686–3689. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, C.; Wang, W.; Che, X.; Cao, Y.; Dong, G.; Wang, S.; Ji, H.; Miao, Z.; Yao, J. Structure-based rational design, synthesis and antifungal activity of oxime-containing azole derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 2942–2945. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Sheng, C.; Wang, W.; Cao, Y.; Xu, Y.; Ji, H.; Dong, G.; Miao, Z.; Yao, J.; Zhang, W. New azoles with potent antifungal activity: Design, synthesis and molecular docking. Eur. J. Med. Chem. 2009, 44, 4218–4226. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, L.; Wang, Y.; Song, Y.; Cao, Y.; Jiang, Y.; Sun, Q.; Wu, Q. Molecular docking, design, synthesis and antifungal activity study of novel triazole derivatives containing the 1,2,3-triazole group. RSC Adv. 2013, 3, 13486–13490. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Badali, H.; Faramarzi, M.A.; Samadi, N.; Afsarian, M.H.; Irannejad, H.; Emami, S. Novel triazole alcohol antifungals derived from fluconazole: Design, synthesis, and biological activity. Mol. Divers. 2014, 19, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef] [PubMed]
- Yehye, W.A.; Abdul Rahman, N.; Saad, O.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M.; Matlob, A.A. Rational design and synthesis of new, high efficiency, multipotent schiff base-1, 2, 4-triazole antioxidants bearing butylated hydroxytoluene moieties. Molecules 2016, 21, 847. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Iram, S.; Thomas, J.; Hayat, M.Q.; Pannecouque, C.; Dehaen, W. Application of the triazolization reaction to afford dihydroartemisinin derivatives with anti-HIV activity. Molecules 2017, 22, 303. [Google Scholar] [CrossRef] [PubMed]
- Giofrè, S.V.; Romeo, R.; Carnovale, C.; Mancuso, R.; Cirmi, S.; Navarra, M.; Garozzo, A.; Chiacchio, M.A. Synthesis and biological properties of 5-(1H-1,2,3-triazol-4-yl)isoxazolidines: A new class of c-nucleosides. Molecules 2015, 20, 5260–5275. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Pistone, A.; Ziccarelli, I.; Espro, C.; Galvagno, S.; Giofré, S.V.; Romeo, R.; Cicero, N.; Bua, G.D.; Lanza, G. Removal of heavy metal ions from wastewaters using dendrimer-functionalized multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2017, 17, 14735–14747. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Pistone, A.; Visco, A.; Galtieri, G.; Giofrè, S.V.; Romeo, R.; Romeo, G.; Cappello, S.; Bonsignore, M.; Denaro, R. 1,2,3-triazole/mwcnt conjugates as filler for gelcoat nanocomposites: New active antibiofouling coatings for marine application. Mater. Res. Express 2015, 2, 115001. [Google Scholar] [CrossRef]
- Mital, A. Synthetic nitroimidazoles: Biological activities and mutagenicity relationships. Sci. Pharm. 2009, 77, 497–520. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Xu, J.M.; Cao, Y.B.; Zhang, W.N.; Wu, Q.Y.; Zhang, D.Z.; Zhang, J.; Zhao, H.Q.; Jiang, Y.Y. Synthesis of novel triazole derivatives as inhibitors of cytochrome p450 14α-demethylase (CYP51). Eur. J. Med. Chem. 2007, 42, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Zhang, J.; Hu, H.; Yu, S.; Sun, Q.; Dan, Z.; Jiang, Y.; Wu, Q. Design, synthesis, and biological evaluation of novel triazole derivatives as inhibitors of cytochrome p450 14α-demethylase. Eur. J. Med. Chem. 2009, 44, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xu, H.; Tian, Y.; Guo, M.; Su, X.; Guo, C. Design, synthesis and antifungal activity of novel benzofuran-triazole hybrids. Molecules 2016, 21, 732. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, U.D.; Tupe, S.G.; Seijas Vazquez, J.A.; Khan, F.A.; Sangshetti, J.N.; Nikalje, A.P.G. Ultrasound-and molecular sieves-assisted synthesis, molecular docking and antifungal evaluation of 5-(4-(Benzyloxy)-substituted phenyl)-3-((phenylamino) methyl)-1,3,4-oxadiazole-2(3H)-thiones. Molecules 2016, 21, 484. [Google Scholar] [CrossRef] [PubMed]
- Borate, H.B.; Maujan, S.R.; Sawargave, S.P.; Chandavarkar, M.A.; Vaiude, S.R.; Joshi, V.A.; Wakharkar, R.D.; Iyer, R.; Kelkar, R.G.; Chavan, S.P. Fluconazole analogues containing 2H-1,4-benzothiazin-3(4H)-one r 2H-1,4-benzoxazin-3(4H)-one moieties, a novel class of anti-candida agents. Bioorg. Med. Chem. Lett. 2010, 20, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. Gromacs: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and autodocktools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Approved Standard Clsi Document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Approved Standard Clsi Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
Sample Availability: Not Available. |

| Tested Fungi | Compounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | 5i | 5j | Fluconazole | |
| C. glabrata (CBS 863) | 128 | 16 | G | G | G | 256 | 8 | 64 | G | G | 4 |
| C. dubliniensis (CBS 8501) | 4 | 0.5 | 128 | 16 | G | G | 32 | G | G | G | 0.5 |
| C. dubliniensis (CBS 7987) | 2 | 0.5 | 32 | 4 | 64 | G | 1 | G | G | G | 0.5 |
| C. dubliniensis (CBS 7988) | 2 | 0.5 | 8 | 2 | 32 | G | 32 | G | G | G | 0.5 |
| C. albicans (CBS 5982) | 256 | 128 | 256 | 256 | G | G | 2 | G | G | G | 256 |
| C. glabrata (CBS 6144) | 128 | 32 | G | G | G | G | 4 | G | G | G | 4 |
| C. albicans (CBS 2730) | 16 | 2 | 256 | 32 | G | G | 64 | G | G | G | <0.5 |
| C. albicans (CBS 562) | 16 | 1 | 128 | 32 | G | G | 16 | G | G | G | <0.5 |
| C. albicans (CBS 1912) | 8 | 0.5 | 128 | 16 | 256 | 16 | 2 | G | G | G | 0.25 |
| C. glabrata (CBS 2192) | 128 | 32 | G | G | G | 32 | 0.5 | G | G | G | 8 |
| C. glabrata (CBS 2175) | 128 | 16 | G | G | G | 32 | 1 | G | G | G | 4 |
| C. dubliniensis (CBS 8500) | 8 | 0.5 | 64 | 8 | 256 | G | 128 | G | G | G | 0.25 |
| C. albicans (CBS 1905) | 4 | <0.5 | 64 | 8 | G | G | 0.5 | G | 128 | G | 0.5 |
| C. tropicalis (ATCC750) | 16 | 4 | G | 64 | G | G | 4 | NT | G | G | 2 |
| C. arapsilosis (ATCC4344) | 16 | 2 | G | 128 | G | G | 8 | NT | 64 | G | 0.5 |
| C. krusei (ATCC6258) | G | 128 | G | G | G | 64 | 1 | NT | 64 | G | G |
| Cryptococcus neoformance (ATCC9011) | 32 | 2 | 256 | 64 | 256 | G | 8 | NT | G | G | 1 |
| C. albicans * | 4 | 0.5 | 128 | 16 | 256 | 64 | 2 | 256 | 256 | 256 | 1 |
| C. glabrata * | NT | NT | NT | NT | NT | 128 | 8 | 256 | 256 | 256 | 0.25 |
| C. dublinien * | 16 | 1 | 256 | 256 | 256 | 16 | 1 | 256 | 128 | 256 | 0.5 |
| Fungi | Compounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | 5i | 5j | Fluconazole | |
| Microsporum gypseum | 64 | 4 | G | G | G | 256 | 64 | 256 | G | G | 8 |
| Epidermophyton floccosum | 128 | 64 | G | G | G | 64 | 32 | 64 | G | G | G |
| Trichophyton mentagrophytes | 128 | 128 | G | G | G | 64 | G | G | G | G | G |
| Aspergillus fumigatus | 128 | G | G | G | G | G | G | G | G | G | G |
| Aspergillus fumigatus | 128 | G | G | G | G | G | G | G | G | G | G |
| Aspergillus fumigatus | G | G | G | G | G | G | G | G | G | G | G |
| Aspergillus clevatus | G | G | G | G | G | G | G | G | G | G | G |
| Aspergillus flavus | G | G | G | G | G | G | G | G | G | G | G |

| Compounds | Final Docked Energy (Kcal/mol) | LogP | R1 | R2 | R3 |
|---|---|---|---|---|---|
| 5a | −10.82 | 0.73 | F | F | 3-nitro-1,2,4-triazol |
| 5b | −11.98 | 1.48 | Cl | Cl | 3-nitro-1,2,4-triazol |
| 5c | −11.91 | 0.59 | F | H | 3-nitro-1,2,4-triazol |
| 5d | −11.83 | 0.97 | Cl | H | 3-nitro-1,2,4-triazol |
| 5e | −11.14 | 0.45 | H | H | 3-nitro-1,2,4-triazol |
| 5f | −7.17 | 0.27 | F | F | 2-(piperazin-1-yl)ethanol |
| 5g | −8.26 | 1.31 | Cl | Cl | 2-(piperazin-1-yl)ethanol |
| 5h | −7.36 | 0.41 | F | H | 2-(piperazin-1-yl)ethanol |
| 5i | −8.03 | 0.79 | Cl | H | 2-(piperazin-1-yl)ethanol |
| 5j | −7.25 | 0.28 | H | H | 2-(piperazin-1-yl)ethanol |
| Fluconazole | −6.74 | 0.99 | F | F | 1H-1,2,4-triazole |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghpour, H.; Khabnadideh, S.; Zomorodian, K.; Pakshir, K.; Hoseinpour, K.; Javid, N.; Faghih-Mirzaei, E.; Rezaei, Z. Design, Synthesis, and Biological Activity of New Triazole and Nitro-Triazole Derivatives as Antifungal Agents. Molecules 2017, 22, 1150. https://doi.org/10.3390/molecules22071150
Sadeghpour H, Khabnadideh S, Zomorodian K, Pakshir K, Hoseinpour K, Javid N, Faghih-Mirzaei E, Rezaei Z. Design, Synthesis, and Biological Activity of New Triazole and Nitro-Triazole Derivatives as Antifungal Agents. Molecules. 2017; 22(7):1150. https://doi.org/10.3390/molecules22071150
Chicago/Turabian StyleSadeghpour, Hossein, Soghra Khabnadideh, Kamiar Zomorodian, Keyvan Pakshir, Khadijeh Hoseinpour, Nabiollah Javid, Ehsan Faghih-Mirzaei, and Zahra Rezaei. 2017. "Design, Synthesis, and Biological Activity of New Triazole and Nitro-Triazole Derivatives as Antifungal Agents" Molecules 22, no. 7: 1150. https://doi.org/10.3390/molecules22071150
APA StyleSadeghpour, H., Khabnadideh, S., Zomorodian, K., Pakshir, K., Hoseinpour, K., Javid, N., Faghih-Mirzaei, E., & Rezaei, Z. (2017). Design, Synthesis, and Biological Activity of New Triazole and Nitro-Triazole Derivatives as Antifungal Agents. Molecules, 22(7), 1150. https://doi.org/10.3390/molecules22071150





