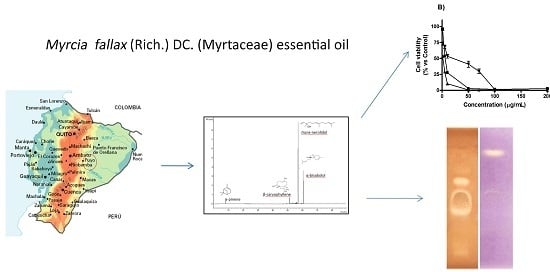
Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Essential Oil
2.2. Cytotoxic Activity
2.3. Antibacterial Activity: HPTLC-Bioautography and MIC
2.4. Antioxidant Activity
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of Essential Oil
3.3. Chemicals
3.4. GC and GC-MS Analysis of the Essential Oil
3.5. In Vitro Cytotoxic Activity
3.6. Antibacterial Activity: HPTLC Bioautographic Assay
3.7. Determination of Minimum Inhibitory Concentration (MIC)
3.8. Antioxidant Properties
3.8.1. Spectrophotometric DPPH Assay
3.8.2. DPPH-HPTLC Bioautography
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Başer, K.H.C.; Buchbauer, G. Introduction. In Handbook of Essential Oils: Science, Technology and Applications, 1st ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 1–2. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Guerrini, A.; Rossi, D.; Grandini, A.; Scalvenzi, L.; Noriega Rivera, P.F.; Andreotti, E.; Tacchini, M.; Spagnoletti, A.; Poppi, I.; Maietti, S.; et al. Biological and chemo-diverse characterization of Amazonian (Ecuador) Citrus petitgrains. J. Appl. Bot. Food Qual. 2014, 87, 108–116. [Google Scholar] [CrossRef]
- Fattahi, B.; Nazeri, V.; Kalantari, S.; Bonfill, M.; Fattahi, M. Essential oil variation in wild-growing populations of a Salvia reuterana Boiss. collected from Iran: Using GC-MS and multivariate analysis. Ind. Crops Prod. 2016, 81, 180–190. [Google Scholar] [CrossRef]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oil: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, L.D.; Peña, A.E.; Gonzales de, C.N.; Quintero, A.; Meza, M.; Usubillaga, A.; Velasco, J. Composition and antibacterial activity of the essential oil of Myrcia fallax (Rich.) DC. from Venezuela. Rev. Soc. Quim. Perú 2009, 75, 221–227. [Google Scholar]
- Stefanello, M.E.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential oils from neotropical Myrtaceae: Chemical diversity and biological properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.M.; Wofor, A.G. Biologically Active Extracts from Myrcia fallax (Myrtaceae) Peru and Method of Obtaining Same. U.S. Patent 4,451,459, 29 May 1984. [Google Scholar]
- Stefanello, M.E.A.; Riva, D.; Simionatto, E.L.; De Carvalho, J.E.; Góis Ruiz, A.L.; Salvador, M.J. Chemical composition and cytotoxic activity of essential oil from Myrcia laruotteana fruits. J. Essent. Oil Res. 2011, 23, 7–10. [Google Scholar] [CrossRef]
- Henriques, A.T.; Sobral, M.; Bridi, R.; Vérin, P.; Menut, C.; Lamaty, G.; Bessiere, J.M. Essential oils from five southern brazilian species of Myrcia (Myrtaceae). J. Essent. Oil Res. 1997, 9, 13–18. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Masspectrometry, 4th ed.; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Stefanello, M.E.A.; Cervi, A.C.; Wisniewski, A., Jr.; Simionatto, E.L. Composição e variação sazonal do oleo essencial de Myrcia obtecta (O. Berg.) Kiaersk. var. obtecta, Myrtaceae. Rev. Bras. Farmacogn. 2010, 20, 82–86. [Google Scholar] [CrossRef]
- Nakamura, M.J.; Monteiro, S.S.; Bizarri, C.H.B.; Siani, A.C.; Ramos, M.F.S. Essential oils of four Myrtaceae species from the Brazilian southeast. Biochem. Syst. Ecol. 2010, 38, 1170–1175. [Google Scholar] [CrossRef]
- Lima, G.S.L.; Zoghbi, M.G.B.; Bastos, M.N.C.; Jardim, M.A.G. Óleos Essenciais de Espécies de Eugenia L. e Myrcia DC. ex Guill. (Myrtaceae) Nativas da Restinga da APA de Algodoal-Maiandeua. In Diversidade Biológica das Áreas de Proteção Ambiental Ilhas do Combu and Maiandeua–Pará; Jardim, M.A.G., Ed.; MPEG: Belem, Brazil, 2009; pp. 405–424. [Google Scholar]
- Pereira, R.A.; Zoghbi, M.G.B.; Bastos, M.N.C. Essential oils of twelve species of Myrtaceae growing wild in the sandbank of the Resex Maracanã, State of Pará, Brazil. J. Essent. Oil Bear. Plants 2010, 13, 440–450. [Google Scholar] [CrossRef]
- Carreira, M.M.; Zoghbi, M.G.B.; Andrade, E.H.A.; da Silva, M.H.L.; Maia, J.G.S. Essential oils from three Myrcia species. Flav. Fragr. J. 2003, 18, 421–424. [Google Scholar] [CrossRef]
- Sá, F.A.S.; Borges, L.L.; Paula, J.A.M.; Sampaio, B.L.; Ferri, P.H.; Paula, J.R. Essential oils in aerial parts of Myrcia tomentosa: Composition and variability. Rev. Bras. Farmacogn. 2012, 22, 1233–1240. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Souza, L.C.; Passos, M.G.; Lima, E.; Poque, N.F.; Martins, D.; Guedes, M.L.S.; Cruz, G.F. Seasonal variation and antimicrobial activity of Myrcia myrtifolia essential oils. J. Braz. Chem. Soc. 2007, 18, 998–1003. [Google Scholar] [CrossRef]
- Padovan, A.; Keszei, A.; Külheim, C.; Foley, W.J. The evolution of foliar terpene diversity in Myrtaceae. Phytochem. Rev. 2014, 13, 695–716. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahasneh, A.M. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci. Pharm. 2010, 78, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Viljoen, A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Seki, T.; Kokuryo, T.; Yokoyama, Y.; Suzuki, H.; Itatsu, K.; Nakagawa, A.; Mizutani, T.; Miyake, T.; Uno, M.; Yamauchi, K.; Nagino, M. Antitumor effects of α-bisabolol against pancreatic cancer. Cancer Sci. 2011, 102, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Costarelli, L.; Malavolta, M.; Giacconi, R.; Cipriano, C.; Gasparini, N.; Tesei, S.; Pierpaoli, S.; Orlando, F.; Suzuki, H.; Perbellini, L.; et al. In vivo effect of α-bisabolol, a non toxic sesquiterpene alcohol, on the induction of spontaneous mammary tumors in HER-2/neu transgenic mice. Oncol. Res. 2009, 18, 409–418. [Google Scholar] [CrossRef]
- Sylvestre, M.; Pichette, A.; Lavoie, S.; Longtin, A.; Legault, J. Composition and cytotoxic activity of the leaf essential oil of Comptonia peregrina (L.). Coulter. Phytother. Res. 2007, 21, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, L.W. Inhibition of azoxymethane-induced neoplasia of the large bowel by 3-hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene (nerolidol). Carcinogenesis 1991, 12, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.; Rocha, S.; Coimbra, M.; Vieira, M. Enhancement of Escherichia coli and Staphylococcus aureus antibiotic susceptibility using sesquiterpenoids. Med. Chem. 2008, 4, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Siciliano, T.; D’Arrigo, M.; Germanò, M.P. Chemical composition and antimicrobial activity of Momordica charantia seed essential oil. Fitoterapia 2008, 79, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kang, S.C. In vitro control of food-borne and food spoilage bacteria by essential oil and ethanol extracts of Lonicera japonica Thunb. Food Chem. 2009, 116, 670–675. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Hamdi, N.; Ben Halima, N. Characterization of essential oil from Citrus aurantium L. flowers: Antimicrobial and antioxidant activities. J. Oleo Sci. 2013, 62, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Khaoukha, G.; Ben Jemia, M.; Smain, A.; Bruno, M.; Scandolera, E.; Senatore, F. Characterization and antimicrobial activity of the volatile components of the flowers of Magydaris tomentosa (Desf.) DC. collected in Sicily and Algeria. Nat. Prod. Res. 2014, 28, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Rustaiyan, A.; Faridchehr, A. A review on constituents and biological activities of further Iranian Artemisia species. Int. J. Pharm. Biol. Chem. Sci. 2014, 3, 6–14. [Google Scholar]
- Sperotto, A.R.M.; Moura, D.J.; Péres, V.F.; Damasceno, F.C.; Caramão, E.B.; Henriques, J.A.P.; Saffi, J. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem. Toxicol. 2013, 57, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nostro, A.; Guerrini, A.; Marino, A.; Tacchini, M.; Di Giulio, M.; Grandini, A.; Akin, M.; Cellini, L.; Bisignano, G.; Saraçoğlu, H.T. In vitro activity of plants extracts against biofilm-producing food-related bacteria. Int. J. Food Microbiol. 2016, 238, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Bladt, S. Plant Drug Analysis. A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin, Germany, 1996; p. 364. [Google Scholar]
- Clinical Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; Approved Standard M07-A9; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Buchbauer, G. Biological activities of essential oils. In Handbook of Essential Oils: Science, Technology and Applications, 1st ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 235–280. [Google Scholar]
- Erdal, M.S.; Peköz, A.Y.; Aksu, B.; Araman, A. Impacts of chemical enhancers on skin permeation and deposition of terbinafine. Pharm. Dev. Technol. 2014, 19, 565–570. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the essential oil of Myrcia splendens are available from the authors. |



| Antioxi. F. 5 | Antibact. F. 6 | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | % Area 1 | Component 2 | AI Exp 3 | AI Lit 4 | Rf = 0.9 | Rf = 0.45–0.6 | Rf = 0.7 | |
| 1 | 2.08 ± 0.10 | α-pinene | 928 | 939 | ||||
| 2 | 0.11 ± 0.01 | β-pinene | 972 | 979 | ||||
| 3 | 0.15 ± 0.02 | α-cubebene | 1350 | 1351 | 1.39 | |||
| 4 | 0.51 ± 0.03 | α-copaene | 1375 | 1377 | 4.21 | |||
| 5 | 0.11 ± 0.02 | β-elemene | 1387 | 1391 | 4.39 | |||
| 6 | 4.21 ± 0.15 | β-caryophyllene | 1409 | 1419 | 36.23 | |||
| 7 | 0.31 ± 0.02 | trans-α-bergamotene | 1430 | 1435 | 3.15 | |||
| 8 | 0.45 ± 0.03 | α-caryophyllene | 1449 | 1455 | 3.94 | |||
| 9 | 0.87 ± 0.06 | trans-β-farnesene | 1453 | 1457 | 7.81 | |||
| 10 | 0.65 ± 0.04 | germacrene D | 1475 | 1485 | 4.62 | |||
| 11 | 0.10 ± 0.01 | cis-β-guaiene | 1485 | 1493 | 1.54 | |||
| 12 | 0.17 ± 0.02 | viridiflorene | 1490 | 1497 | 1.43 | |||
| 13 | 0.11 ± 0.01 | α-muurolene | 1497 | 1499 | 1.27 | |||
| 14 | 0.59 ± 0.03 | α-bisabolene | 1503 | 1507 | 1.24 | |||
| 15 | 0.24 ± 0.02 | cis-γ-bisabolene | 1506 | 1515 | 8.33 | |||
| 16 | 0.53 ± 0.04 | δ-cadinene | 1513 | 1523 | 4.93 | |||
| 17 | 0.16 ± 0.01 | trans-calamenene | 1518 | 1529 | 1.66 | |||
| 18 | 1.03 ± 0.09 | trans-γ-bisabolene | 1523 | 1531 | 10.04 | |||
| 19 | 67.81 ± 2.10 | trans-nerolidol | 1562 | 1563 | 100 | |||
| 20 | 0.15 ± 0.01 | caryophyllene oxide | 1580 | 1583 | ||||
| 21 | Traces | β-cedren-9-one | 1630 | 1631 | 87.34 | |||
| 22 | 17.51 ± 1.01 | α-bisabolol | 1690 | 1686 | ||||
| Total identified | 97.84 | |||||||
| Cell Line (IC50 µg/mL) 1 | |||
|---|---|---|---|
| A549 2 | MCF-7 3 | HaCaT 4 | |
| M. splendens EO | 100.99 ± 2.32 6d | 5.59 ± 0.13 6c | 21.58 ± 1.26 6c |
| α-bisabolol | 27.63 ± 2.01 6b | 1.24 ± 0.03 6a | 10.15 ± 0.35 6b |
| trans-nerolidol | 54.28 ± 2.39 6c | 40.97 ± 5.07 6d | 27.76 ± 2.76 6d |
| doxorubicin 5 | 0.90 ± 0.01 6a | 2.10 ± 0.42 6b | 0.40 ± 0.01 6a |
| Strain | M. splendens EO 1 | Antibiotic 2 | |
|---|---|---|---|
| Gram negative | |||
| Agrobacterium tumefaciens | DSM 30207 | 500 3c | 6.25 3c |
| Agrobacterium vitis | DSM 6583 | 2000 3e | 1.56 3a |
| Pseudomonas syringae pv. syringae | DSM 10604 | 250 3b | 3.12 3b |
| Escherichia coli | ATCC 4350 | >2000 3f | 25.00 3d |
| Pseudomonas aeruginosa | ATCC 27853 | >2000 3f | 6.25 3c |
| Gram positive | |||
| Clavibacter michiganensis subsp. nebraskensis | DSM 20400 | 125 3a | 6.25 3c |
| Enterococcus faecalis | ATCC 29212 | 2000 3e | 3.12 3b |
| Listeria grayi | DSM 20601 | 1000 3d | 5.00 3c |
| Staphylococcus aureus | ATCC 29230 | 1000 3d | 3.12 3b |
| Staphylococcus epidermidis | ATCC 14990 | 1000 3d | 3.12 3b |
| Sample | IC50 (μg/mL) 1 |
|---|---|
| M. splendens EO | 43,537.00 ± 15 3b |
| vitamin E 2 | 7.8 ± 0.5 3a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalvenzi, L.; Grandini, A.; Spagnoletti, A.; Tacchini, M.; Neill, D.; Ballesteros, J.L.; Sacchetti, G.; Guerrini, A. Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile. Molecules 2017, 22, 1163. https://doi.org/10.3390/molecules22071163
Scalvenzi L, Grandini A, Spagnoletti A, Tacchini M, Neill D, Ballesteros JL, Sacchetti G, Guerrini A. Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile. Molecules. 2017; 22(7):1163. https://doi.org/10.3390/molecules22071163
Chicago/Turabian StyleScalvenzi, Laura, Alessandro Grandini, Antonella Spagnoletti, Massimo Tacchini, David Neill, José Luis Ballesteros, Gianni Sacchetti, and Alessandra Guerrini. 2017. "Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile" Molecules 22, no. 7: 1163. https://doi.org/10.3390/molecules22071163