Synthesis, Physico-chemical Characterization, Crystal Structure and Influence on Microbial and Tumor Cells of Some Co(II) Complexes with 5,7-Dimethyl-1,2,4-triazolo[1,5-a]pyrimidine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Complexes
2.2. Characterization of the Complexes
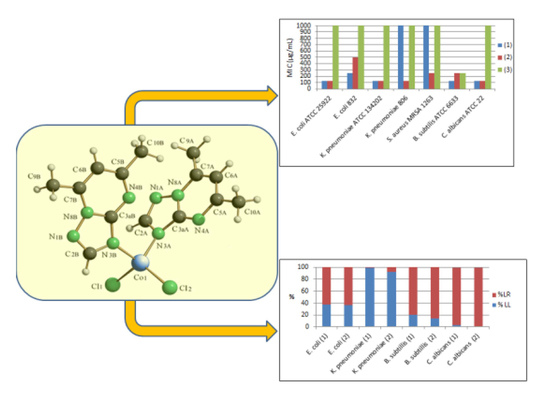
2.2.1. Description of the X-ray Crystal Structures of the Complexes
2.2.2. Infrared Spectra
2.2.3. UV-Vis-NIR Spectra and Magnetic Moments
2.3. Biological Activity
2.3.1. Antimicrobial Activity
2.3.2. In Vitro Assessment of the Complexes Influence on the HEp-2 Cellular Cycle and Gene Expression
3. Experimental Section
3.1. General Information
3.2. Synthesis of Complexes
3.2.1. [Co2(dmtp)2(OH2)4][CoCl4] (1)
3.2.2. [Co(dmtp)2Cl2] (2)
3.2.3. [Co(dmtp)2(OH2)4]Cl2·2H2O (3)
3.3. X-ray Crystallography
3.4. Biological Assays
3.4.1. Screening of the Antimicrobial Properties
3.4.2. Mammalian Cells Morphology and Apoptosis Evaluation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, T.; Kolodkin-Gal, I.; Kolter, R.; Losick, R.; Clardy, J. Synthesis and Activity of Biomimetic Biofilm Disruptors. J. Am. Chem. Soc. 2013, 135, 2927–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, V. Microbial Adherence; Romanian Academy Publishing House: Bucharest, Romania, 2003. [Google Scholar]
- Said, S.A.; Amr, A.-G.; Sabry, N.M.; Abdalla, M.M. Analgesic, anticonvulsant and anti-inflammatory activities of some synthesized benzodiazipine, triazolopyrimidine and bis-imide derivatives. Eur. J. Med. Chem. 2009, 44, 4787–4792. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Thabet, H.K.H.; Helal, M.H.; Abdelaal, A.S.; Ammar, Y.A. Synthesis and Pharmacological Evaluation of Some Pyrazoles, Thiazolopyrimidine, Triazolopyrimidine, Pyridone and 2- Iminochromene Containing Naproxenoyl Moiety as NSAIDs. Chem. Sci. J. 2011, 32, 2150–3494. [Google Scholar]
- Ashour, H.M.; Shaaban, O.G.; Rizk, O.H.; El-Ashmawy, I.M. Synthesis and biological evaluation of thieno [2′,3′: 4,5] pyrimido [1,2-b][1,2,4] triazines and thieno [2,3-d][1,2,4] triazolo [1,5-a] pyrimidines as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2013, 62, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.D.; Chudasama, C.J.; Patel, K.D. Pyrazole clubbed triazolo [1,5-a] pyrimidine hybrids as an anti-tubercular agents: Synthesis, in vitro screening and molecular docking study. Bioorg. Med. Chem. 2015, 23, 7711–7716. [Google Scholar] [CrossRef] [PubMed]
- Boechat, N.; Pinheiro, L.C.S.; Silva, T.S.; Aguiar, A.C.C.; Carvalho, A.S.; Bastos, M.M.; Costa, C.C.P.; Pinheiro, S.; Pinto, A.C.; Mendonça, J.S.; et al. New Trifluoromethyl Triazolopyrimidines as Anti-Plasmodium falciparum Agents. Molecules 2012, 17, 8285–8302. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, Y.; Chen, W.; Liu, H.; Zhan, P.; Li, D.; Liu, H.; De Clercq, E.; Pannecouque, C.; Liu, X. Fused heterocycles bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 2: Discovery of novel [1,2,4]Triazolo[1,5-a]pyrimidines using a structure-guided core-refining approach. Eur. J. Med. Chem. 2014, 85, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ayral-Kaloustian, S.; Nguyen, T.; Afragola, J.; Hernandez, R.; Lucas, J.; Gibbons, J.; Beyer, C. Synthesis and SAR of [1,2,4]Triazolo[1,5-a]pyrimidines, a Class of Anticancer Agents with a Unique Mechanism of Tubulin Inhibition. J. Med. Chem. 2007, 50, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Faizi, M.; Dabirian, S.; Tajali, H.; Ahmadi, F.; Zavareh, E.R.; Shahhosseini, S.; Tabatabai, S.A. Novel agonists of benzodiazepine receptors: Design, synthesis, binding assay and pharmacological evaluation of 1,2,4-triazolo [1,5-a] pyrimidinone and 3-amino-1,2,4-triazole derivatives. Bioorg. Med. Chem. 2015, 23, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Novinson, T.; Springer, R.H.; O’Brien, D.E.; Scholten, M.B.; Miller, J.P.; Robins, R.K. 2-(Alkylthio)-1,2,4-triazolo[1,5-a]pyrimidines as adenosine cyclic 3′,5′-monophosphate phosphodiesterase inhibitors with potential as new cardiovascular agents. J. Med. Chem. 1982, 25, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Ruisi, G.; Canfora, L.; Bruno, G.; Rotondo, A.; Mastropietro, T.F.; Debbia, E.A.; Girasolo, M.A.; Megna, B. Triorganotin(IV) derivatives of 7-amino-2-(methylthio)[1,2,4]triazolo[1,5-a]pyrimidine-6-carboxylic acid. Synthesis, spectroscopic characterization, in vitro antimicrobial activity and X-ray crystallography. J. Organomet. Chem. 2010, 695, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Girasolo, M.A.; Canfora, L.; Sabatino, P.; Schillaci, D.; Foresti, E.; Rubino, S.; Ruisi, G.; Stocco, G. Synthesis, characterization, crystal structures and in vitro antistaphylococcal activity of organotin(IV) derivatives with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidine. J. Inorg. Biochem. 2012, 106, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, M.A.; Salas, J.M.; Quirós, M.; Molina, J. Cobalt(II) complexes of 5,7-dimethyl[1,2,4]-triazolo-[1,5-a]-pyrimidine. Spectroscopic characterization, XRD study and antimicrobial activity. Trans. Met. Chem. 1993, 18, 595–598. [Google Scholar] [CrossRef]
- Bavelaar, K.; Khalil, R.; Mutikainen, I.; Turpeinen, U.; Marquès-Gallego, P.; Kraaijkamp, M.; van Albada, G.A.; Haasnoot, J.G.; Reedijk, J. A dinuclear silver compound with 5,6,7-trimethyl-[1,2,4]triazolo[1,5-a]pyrimidine with a short Ag–Ag bond. Synthesis, characterization, single-crystal structure analysis and cytostatic activity. Inorg. Chim. Acta 2011, 366, 81–84. [Google Scholar] [CrossRef]
- Girasolo, M.A.; Attanzio, A.; Sabatino, P.; Tesoriere, L.; Rubino, S.; Stocco, G. Organotin(IV) derivatives with 5,7-disubstituted-1,2,4-triazolo [1,5-a]pyrimidine and their cytotoxic activities: The importance of being conformers. Inorg. Chim. Acta 2014, 423, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Łakomska, I.; Fandzloch, M.; Wojtczak, A. Dimeric ruthenium-triazolopyrimidine complex: Synthesis and structural characterization. Inorg. Chem. Commun. 2014, 49, 24–26. [Google Scholar] [CrossRef]
- Łakomska, I.; Wojtczak, A.; Sitkowski, J.; Kozerski, L.; Szłyk, E. Platinum (IV) complexes with purine analogs. Studies of molecular structure and antiproliferative activity in vitro. Polyhedron 2008, 27, 2765–2770. [Google Scholar] [CrossRef]
- Łakomska, I.; Hoffmann, K.; Topolski, A.; Kloskowski, T.; Drewa, T. Spectroscopic, kinetic and cytotoxic in vitro study of hexafluoroglutarate platinum(II) complex with 5,7-dimethyl-1,2,4-triazolo[1,5-a] pyrimidine. Inorg. Chim. Acta 2012, 387, 455–459. [Google Scholar] [CrossRef]
- Rubino, S.; Di Stefano, V.; Attanzio, A.; Tesoriere, L.; Girasolo, M.A.; Nicolo, F.; Bruno, G.; Orecchio, S.; Stocco, G.C. Synthesis, spectroscopic characterization and antiproliferative activity of two platinum(II) complexes containing N-donor heterocycles. Inorg. Chim. Acta 2014, 418, 112–148. [Google Scholar] [CrossRef]
- Łakomska, I.; Hoffmann, K.; Wojtczak, A.; Sitkowski, J.; Maj, E.; Wietrzyk, J. Cytotoxic malonateplatinum(II) complexes with 1,2,4-triazolo[1,5-a]pyrimidine derivatives: Structural characterization and mechanism of the suppression of tumor cell growth. J. Inorg. Biochem. 2014, 141, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Łakomska, I.; Wiśniewska, J.; Kaczmarek-Kędziera, A.; Wietrzyk, J. Acetate platinum(II) compound with 5,7-ditertbutyl-1,2,4-triazolo[1,5-a]pyrimidine that overcomes cisplatin resistance: structural characterization, in vitro cytotoxicity, and kinetic studies. J. Coord. Chem. 2015, 68, 3193–3208. [Google Scholar] [CrossRef]
- Łakomska, I.; Babinska, M.; Wojtczak, A.; Sitkowski, J. Synthesis, characterization and in vitro cytotoxicity of three types of platinum(II) complexes containing 5,7-diethyl-1,2,4-triazolo[1,5-a]pyrimidine. Inorg. Chim. Acta 2016, 453, 516–521. [Google Scholar] [CrossRef]
- Wiśniewska, K.H.J.; Wojtczak, A.; Sitkowskic, J.; Denslow, A.; Wietrzyk, J.; Jakubowski, M.; Łakomska, I. Rational design of dicarboxylato platinum(II) complexes with purine-mimetic ligands as novel anticancer agents. J. Inorg. Biochem. 2017, 172, 34–45. [Google Scholar]
- Caballero, A.B.; Rodríguez-Dieguez, A.; Quirós, M.; Salas, J.M.; Huertas, Ó.; Ramírez-Macías, I.; Olmo, F.; Marín, C.; Chaves-Lemaur, G.; Gutiérrez-Sánchez, R.; et al. Triazolopyrimidine compounds containing first-row transition metals and their activity against the neglected infectious Chagas disease and leishmaniasis. Eur. J. Med. Chem. 2014, 85, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.B.; Marin, C.; Ramírez-Macias, I.; Rodríguez-Dieguez, A.; Quirós, M.; Salas, J.M.; Sánchez-Moreno, M. Structural Consequences of the Introduction of 2,2’-Bipyrimidine as Auxiliary Ligand in Triazolopyrimidine-Based Transition Metal Complexes. In vitro Antiparasitic Activity. Polyhedron 2012, 33, 137–144. [Google Scholar] [CrossRef]
- Łakomska, I.; Fandzloch, M. Application of 1,2,4-triazolo[1,5-a]pyrimidines for the design of coordination compounds with interesting structures and new biological properties. Coord. Chem. Rev. 2016, 327, 221–241. [Google Scholar] [CrossRef]
- Salas, J.M.; Enrique, C.; Romero, M.; Takagi, K.; Aoki, K.; Suh, H. Synthesis and spectroscopic properties of metal complexes of 5,7-dimethyl[1,2,4]triazolo[1,5-a] pyrimidine. X-ray structure of the cobalt(II) and cadmium(II) complexes. Polyhedron 1992, 22, 2903–2912. [Google Scholar] [CrossRef]
- Odabasoglu, M.; Büyükgüngör, O. 5,7-Dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. Acta Crystallogr. 2006, E62, o1310–o1311. [Google Scholar]
- Murphy, B.; Aljabri, M.; Ahmed, A.M.; Murphy, G.; Hathaway, B.J.; Light, M.E.; Geilbrich, T.; Hursthouse, M.B. Structural systematics of the [Cu(chelate)3][Y]2 series. An interesting crystallographic structural insight involving vibronic coupling and the Jahn-Teller effect (JTE). The syntheses and low temperature crystal structures of tris(2,2′bipyridyl)copper(II) tetraphenylborate and tris(2,2′bipyridyl)zinc(II) tetraphenylborate. Dalton Trans. 2006, 14, 357–367. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Gispert, J.R. Coordination Chemistry; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Rice, L.B. Progress and challenges in implementing the research on ESKAPE pathogens. Infect. Control Hosp. Epidemiol. 2010, 31, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Kriengkauykiat, J.; Ito, J.I.; Dadwal, S.S. Epidemiology and treatment approaches in management of invasive fungal infections. Clin. Epidemiol. 2011, 3, 175–191. [Google Scholar] [PubMed]
- Chapeland-Leclerc, F.; Hennequin, C.; Papon, N.; Noël, T.; Girard, A.; Socié, G.; Ribaud, P.; Lacroix, C. Acquisition of flucytosine, azole, and caspofungin resistance in Candida glabrata bloodstream isolates serially obtained from a hematopoietic stem cell transplant recipient. Antimicrob. Agents Chemother. 2010, 54, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Alias, M.F.; Seewan, A.N.; Shakir, C.; Mohammad, F.I. Cytotoxicity Assay of Nickel and Cobalt (II) Complexes of 5-(4-Nitro Phenyl)-4-Amino-3-Mercapto Propenyl-1,2,4-Triazole on HepG2 Cell Line. Int. J. Pharm. 2014, 4, 126–132. [Google Scholar]
- Ahmad, M.; Afzal, M.; Tabassum, S.; Kalińska, B.; Mrozinski, J.; Bharadwaj, P.K. Synthesis and structure elucidation of a cobalt(II) complex as topoisomerase I inhibitor: In vitro DNA binding, nuclease and RBC hemolysis. Eur. J. Med. Chem. 2014, 74, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.-P.; Qin, J.-L.; Meng, T.; Lin, W.-H.; Zhang, C.-H.; Wei, Z.-Z.; Chen, J.-N.; Liu, Y.-C.; Liang, H.; Chen, Z.-F. High in vivo antitumor activity of cobalt oxoisoaporphine complexes by targeting G-quadruplex DNA, telomerase and disrupting mitochondrial functions. Eur. J. Med. Chem. 2016, 124, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Morcelli, S.R.; Bull, É.S.; Terra, W.S.; Moreira, R.O.; Borges, F.V.; Kanashiro, M.M.; Bortoluzzi, A.J.; Maciel, L.L.; de A Almeida, J.C.; Júnior, A.H.; et al. Synthesis, characterization and antitumoral activity of new cobalt(II) complexes: Effect of the ligand isomerism on the biological activity of the complexes. J. Inorg. Biochem. 2016, 161, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Dhayabaran, V.V.; Prakash, T.D.; Renganathan, R.; Friehs, E.; Bahnemann, D.W. Novel Bioactive Co(II), Cu(II), Ni(II) and Zn(II) Complexes with Schiff Base Ligand Derived from Histidine and 1,3-Indandione: Synthesis, Structural Elucidation, Biological Investigation and Docking Analysis. J. Fluoresc. 2017, 27, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Lenstra, A.T.H.; Bruins Slot, H.J.; Beurskens, P.T.; Haasnoot, J.G.; Reedijk, J. Transition metal compounds of 5,7-dimethyl(1,2,4)triazolo(1,5-a)pyrimidine (dmtp). X-ray structures of trans-tetraaquobis (dmtp-N3)nickel(II) diiodide dihydrate and trans-diaquotetrakis(dmtp-N3)nickel(II) bis(triiodide)bis-dmtp. Recl. Trav. Chim. PaysBas 1989, 108, 133–138. [Google Scholar] [CrossRef]
- Hooft, R.W.W. COLLECT, Program for Collecting Data on CCD Area Detectors; Nonius B. V.: Delft, The Netherlands, 1998. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [PubMed]
- Otwinowski, Z.; Borek, D.; Majewski, W.; Minor, W. Multiparametric scaling of diffraction intensities. Acta Crystallogr. 2003, A59, 228–234. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- XP–Interactive Molecular Graphics, Version 5.1; Bruker AXS Inc.: Madison, WI, USA, 1998.
- Olar, R.; Badea, M.; Marinescu, D.; Chifiriuc, M.C.; Bleotu, C.; Grecu, M.N.; Iorgulescu, E.E.; Lazar, V. N,N-Dimethylbiguanide complexes displaying low cytotoxicity as potential large spectrum antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Hawley, R.G.; Hawley, T.S. Flow Cytometry Protocols, 2nd ed.; Humana Press: New York, NY, USA, 2004. [Google Scholar]
- Holban, A.M.; Bleotu, C.; Chifiriuc, M.C.; Bezirtzoglou, E.; Lazar, V. Role of Pseudomonas aeruginosa quorum sensing (QS) molecules on the viability and cytokine profile of human mesenchymal stem cells. Virulence 2014, 5, 303–310. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all compounds are available from the authors. |












| Compound | [Co(dmtp)2(OH2)4][CoCl4] (1) | [Co(dmtp)2Cl2] (2) | [Co(dmtp)2(OH2)4]Cl2·2H2O (3) |
|---|---|---|---|
| Color/shape | Turquoise/plate | Blue/plate | Yellow/plate |
| Empirical formula | C14H24Cl4Co2N8O4 | C14H16Cl2CoN8 | C14H28Cl2CoN8O6 |
| Formula weight | 628.07 | 426.18 | 534.27 |
| Temperature | 223(2) K | 223(2) K | 223(2) K |
| Crystal system | monoclinic | orthorhombic | triclinic |
| Space group | C2/c | Pbca | P |
| Unit cell dimensions | a = 11.4180(2) b = 11.9909(3) Å c = 17.9687(4) Å β = 102.643(1)° | a =10.5555(1) Å b =18.2855(2) Å c =18.4002(2) Å | a =7.6872(4) Å b =8.3348(8) Å c =9.6388(9) Å α = 99.636(8)° β = 100.828(7)° γ = 96.026(4)° |
| Volume | 2400.48(9) Å3 | 3551.47(6) Å3 | 592.05(9) Å3 |
| Z | 4 | 8 | 1 |
| Calculated density | 1.738 mg/m3 | 1.594 mg/m3 | 1.498 mg/m3 |
| Absorption coefficient | 1.865 mm−1 | 1.283 mm−1 | 8.169 mm−1 |
| F(000) | 1272 | 1736 | 277 |
| Goodness-of-fit on F2 | 1.050 | 1.047 | 1.079 |
| Final R indices [I > 2σ(I)] | R1 = 0.0366, wR2 = 0.0747 | R1 = 0.0333, wR2 = 0.0762 | R1 = 0.0433, wR2 = 0.1114 |
| R indices (all data) | R1 = 0.0460, wR2 = 0.0804 | R1 = 0.0411, wR2 = 0.0811 | R1 = 0.0470, wR2 = 0.1149 |
| Largest difference peak and hole | 0.388 and −0.333 e.Å−3 | 0.364 and −0.298 e.Å−3 | 0.274 and −0.406 e.Å−3 |
| Compound | Assignments | |||
|---|---|---|---|---|
| Dmtp | (1) | (2) | (3) | |
| - | 3543 s 3398 vs | - | 3415 w3314 w | ν(OH2) |
| 3101 m | 3116 m | 3119 w | 3123 w | ν(CH) |
| 2918 w | 2950 w | 2920 w | 2955 w | νas(CH3) |
| 2833 w | 2883 w | 2859 w | 2886 w | νs(CH3) |
| 1635 s 1551 vs | 1627 vs 1557 vs | 1625 s 1553 s | 1624 s 1553 vs | ν(C=N) |
| 1445 m | 1444 m | 1426 m | 1441 m | ν(C=C) + δas(CH3) |
| 1038 w | 1031 w | 1040 w | 1039 w | ν(N–N) |
| 810 w | 863 w | 856 w | 891 w | γ(CH) |
| - | 778 w | - | 779 w | ρ(H2O) |
| - | 662 w | - | 662 w | ρw(H2O) |
| - | 573 w | - | 535 w | ν(Co–O) |
| - | 492 w | 487 w | 489 w | ν(Co–N) |
| Compound | Absorption Maxima (cm−1) | Assignment | Magnetic Moment (B.M.) |
|---|---|---|---|
| dmtp | 37,040 33,330 27,025 | π→π* | - |
| [Co(dmtp)2(OH2)4][CoCl4] (1) | 37,740 34,480 | π→π* | 4.47 |
| 19,400 | 4T1g→4T1g(P) | ||
| 15,625 15,040 | 4A2→4T1(P) | ||
| 8440 | 4T1g→4T2g | ||
| 6310 6100 5500 | 4A2→4T1(F) | ||
| [Co(dmtp)2Cl2] (2) | 34,485 | π→π* | 4.29 |
| 15,870 15,150 | 4A2→4T1(P) | ||
| 8930 6830 5900 | 4A2→4T1(F) | ||
| [Co(dmtp)2(OH2)4]Cl2∙2H2O (3) | 38,460 30,765 | π→π* | 5.00 |
| 20,620 | 4T1g→4T1g(P) | ||
| 8625 7510 | 4T1g→4T2g |
| Strain | Complex | ||
|---|---|---|---|
| (1) | (2) | (3) | |
| E. coli ATCC 25922 | inhibition 62.50 | inhibition 62.50 | - |
| E. coli 832 | inhibition 62.50 | inhibition 250 | - |
| K. pneumoniae ATCC 134202 | stimulation | inhibition 62.50 | - |
| K. pneumoniae 806 | - | inhibition 62.50 | - |
| S. aureus MRSA 1263 | - | inhibition 125 | - |
| B. subtilis ATCC 6633 | inhibition 62.50 | inhibition 125 | inhibition 250 |
| C. albicans ATCC 22 | inhibition 62.50 | inhibition 62.50 | - |
| Species | Necrotic Cells | Dead Cells | Early Apoptotic Cells | Viable Cells |
|---|---|---|---|---|
| HEp-2 | 0.56 | 0.28 | 3.15 | 96.00 |
| dmtp | 2.37 | 2.27 | 3.14 | 92.20 |
| (1) | 11.30 | 2.85 | 6.98 | 78.90 |
| (2) | 7.54 | 16.10 | 20.40 | 55.90 |
| (3) | 6.19 | 10.80 | 16.90 | 66.20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Măruţescu, L.; Calu, L.; Chifiriuc, M.C.; Bleotu, C.; Daniliuc, C.-G.; Fălcescu, D.; Kamerzan, C.M.; Badea, M.; Olar, R. Synthesis, Physico-chemical Characterization, Crystal Structure and Influence on Microbial and Tumor Cells of Some Co(II) Complexes with 5,7-Dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. Molecules 2017, 22, 1233. https://doi.org/10.3390/molecules22071233
Măruţescu L, Calu L, Chifiriuc MC, Bleotu C, Daniliuc C-G, Fălcescu D, Kamerzan CM, Badea M, Olar R. Synthesis, Physico-chemical Characterization, Crystal Structure and Influence on Microbial and Tumor Cells of Some Co(II) Complexes with 5,7-Dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. Molecules. 2017; 22(7):1233. https://doi.org/10.3390/molecules22071233
Chicago/Turabian StyleMăruţescu, Luminiţa, Larisa Calu, Mariana Carmen Chifiriuc, Coralia Bleotu, Constantin-Gabriel Daniliuc, Denisa Fălcescu, Crina Maria Kamerzan, Mihaela Badea, and Rodica Olar. 2017. "Synthesis, Physico-chemical Characterization, Crystal Structure and Influence on Microbial and Tumor Cells of Some Co(II) Complexes with 5,7-Dimethyl-1,2,4-triazolo[1,5-a]pyrimidine" Molecules 22, no. 7: 1233. https://doi.org/10.3390/molecules22071233
APA StyleMăruţescu, L., Calu, L., Chifiriuc, M. C., Bleotu, C., Daniliuc, C.-G., Fălcescu, D., Kamerzan, C. M., Badea, M., & Olar, R. (2017). Synthesis, Physico-chemical Characterization, Crystal Structure and Influence on Microbial and Tumor Cells of Some Co(II) Complexes with 5,7-Dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. Molecules, 22(7), 1233. https://doi.org/10.3390/molecules22071233