Astragalosidic Acid: A New Water-Soluble Derivative of Astragaloside IV Prepared Using Remarkably Simple TEMPO-Mediated Oxidation
Abstract
:1. Introduction
2. Results and Discussion
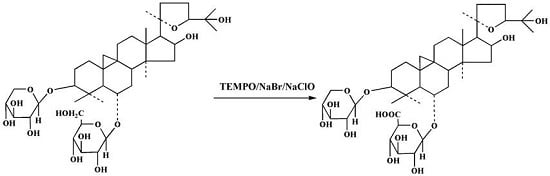
2.1. Preparation of Compound 1
2.2. Structure Elucidation of Compound 1
2.3. Investigation of Cardioprotective Activity In Vitro
3. Materials and Methods
3.1. Apparatus and Materials
3.2. Acid Hydrolysis of Compound 1
3.3. Bioactivity Evaluation
3.3.1. Cell Culture and In Vitro Cardiomycyte Injury Model Induced by Hypoxia/Reoxygenation (H/R)
3.3.2. Cell and Mitochondrial Viability Assay
3.3.3. Creatine Kinase and Lactate Dehydrogenase Activity Detection
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China, 2010 ed.; China Medical Science Press: Beijing, China, 2015; Volume 1, pp. 302–303. ISBN 9787506773379. [Google Scholar]
- Li, L.; Hou, X.; Xu, R.; Tu, M.; Liu, C. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2016, 31, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhang, H.; Mu, Y.P.; Sun, M.Y.; Liu, P. Pharmacological effects of astragaloside IV: A literature review. J. Tradit. Chin. Med. 2013, 33, 413–416. [Google Scholar] [CrossRef]
- Huang, C.R.; Wang, G.J.; Wu, X.L.; Li, H.; Xie, H.T.; Lv, H.; Sun, J.G. Absorption enhancement study of astragaloside IV based on its transport mechanism in caco-2 cells. Eur. J. Drug Metab. Pharmacokinet. 2006, 31, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.C.; Wang, G.J.; Pan, G.Y.; Fawcett, J.P.; Jiye, A.; Sun, J.G. Transport and bioavailability studies of astragaloside IV, an active ingredient in Astragali Radix. Basic Clin. Pharmacol. Toxicol. 2004, 95, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, A.T. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Bragd, P.L.; Besemer, A.C.; Van Bekkum, H. Bromide-free TEMPO-mediated oxidation of primary alcohol groups in starch and methyl alpha-d-glucopyranoside. Carbohydr. Res. 2000, 328, 355–363. [Google Scholar] [CrossRef]
- Qiu, W.L.; Wang, Z.F.; Huang, L.J. Progress on 2,2,6,6-tetramethylpiperineoxyl-mediated oxidationof the primary alcohol of carbohydrate. Chemistry 2007, 70, 29–33. [Google Scholar]
- Vogler, T.; Studer, A. Applications of TEMPO in synthesis. Synthesis 2008, 1979–1993. [Google Scholar]
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.M.; Michaud, P. TEMPO-mediated oxidation of polysaccharides: An ongoing story. Carbohydr. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Wang, H.K.; Takagi, A.; Fuchida, M.; Miura, I.; Yoshikawa, M. Saponin and sapogenol. XXXV. chemical constituents of Astragali Radix, the root of Astragalus membranaceus Bunge. (2). astragalosides I, II and IV, acetylastragaloside I and isoastragalosides I and II. Chem. Pharm. Bull. 1983, 31, 698–708. [Google Scholar] [CrossRef]
- Dong, G.; Chen, T.; Ren, X.; Zhang, Z.F.; Huang, W.; Liu, L.; Luo, P.; Zhou, H. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion 2016, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound 1 is available from the authors. |




| Position | δC | DEPT | Position | δC | DEPT |
|---|---|---|---|---|---|
| 1 | 28.7 | CH2 | 23 | 26.2 | CH2 |
| 2 | 30.0 | CH2 | 24 | 81.4 | CH |
| 3 | 88.2 | CH | 25 | 71.1 | C |
| 4 | 42.4 | C | 26 | 28.4 | CH3 |
| 5 | 52.2 | CH | 27 | 28.5 | CH3 |
| 6 | 79.0 | CH | 28 | 19.6 | CH3 |
| 7 | 34.6 | CH2 | 29 | 28.3 | CH3 |
| 8 | 45.4 | CH | 30 | 16.6 | CH3 |
| 9 | 20.8 | C | |||
| 10 | 27.9 | C | d-xylosyl | ||
| 11 | 25.9 | CH2 | 1′ | 107.4 | CH |
| 12 | 33.2 | CH2 | 2′ | 75.4 | CH |
| 13 | 44.8 | C | 3′ | 78.3 | CH |
| 14 | 45.9 | C | 4′ | 71.0 | CH |
| 15 | 46.0 | CH2 | 5′ | 66.8 | CH2 |
| 16 | 73.2 | CH | d-glucuronyl | ||
| 17 | 58.0 | CH | 1″ | 105.4 | CH |
| 18 | 20.9 | CH3 | 2″ | 75.3 | CH |
| 19 | 32.0 | CH2 | 3″ | 78.2 | CH |
| 20 | 87.0 | C | 4″ | 73.0 | CH |
| 21 | 26.9 | CH3 | 5″ | 77.1 | CH |
| 22 | 34.6 | CH2 | 6″ | 172.7 | C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qing, L.-S.; Peng, S.-L.; Liang, J.; Ding, L.-S. Astragalosidic Acid: A New Water-Soluble Derivative of Astragaloside IV Prepared Using Remarkably Simple TEMPO-Mediated Oxidation. Molecules 2017, 22, 1275. https://doi.org/10.3390/molecules22081275
Qing L-S, Peng S-L, Liang J, Ding L-S. Astragalosidic Acid: A New Water-Soluble Derivative of Astragaloside IV Prepared Using Remarkably Simple TEMPO-Mediated Oxidation. Molecules. 2017; 22(8):1275. https://doi.org/10.3390/molecules22081275
Chicago/Turabian StyleQing, Lin-Sen, Shu-Lin Peng, Jian Liang, and Li-Sheng Ding. 2017. "Astragalosidic Acid: A New Water-Soluble Derivative of Astragaloside IV Prepared Using Remarkably Simple TEMPO-Mediated Oxidation" Molecules 22, no. 8: 1275. https://doi.org/10.3390/molecules22081275
APA StyleQing, L.-S., Peng, S.-L., Liang, J., & Ding, L.-S. (2017). Astragalosidic Acid: A New Water-Soluble Derivative of Astragaloside IV Prepared Using Remarkably Simple TEMPO-Mediated Oxidation. Molecules, 22(8), 1275. https://doi.org/10.3390/molecules22081275