An Introduction to Zeolite Synthesis Using Imidazolium-Based Cations as Organic Structure-Directing Agents
Abstract
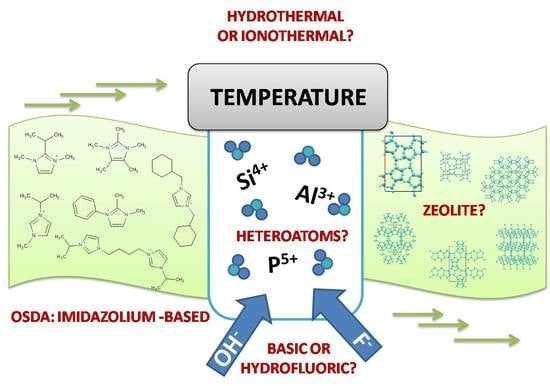
:1. Introduction
2. Zeolites
2.1. Nomenclature and Classification
2.2. Construction
3. Zeotypes
- SM I: substitution of the aluminum bound to phosphorous tetrahedra by monovalent (SM Ia), divalent (SM Ib) or trivalent (SM Ic) elements, resulting in M-O-P bonds.
- SM II: substitution of the phosphorous bound to aluminum tetrahedra by tetravalent (SM IIa) or pentavalent (SM IIb) elements, resulting in M-O-Al bonds.
- SM III: substitution of adjacent aluminum and phosphorous tetrahedra for two silicon tetrahedra.
4. Some Concepts on the Synthesis of Zeolites and Zeotypes
5. Imidazolium Ions in Synthesis of Zeolites and Zeotypes
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cronstedt, A. Beschreibung und untersuchung einer unbekannten Bergart, Zheolites gennant. Abh. Schwed. Akad. Wiss. 1756, 18, 111–113. [Google Scholar]
- Davis, M.E. Zeolites from a materials chemistry perspective. Chem. Mater. 2014, 26, 239–245. [Google Scholar] [CrossRef]
- Kang, J.H.; Xie, D.; Zones, S.I.; Smeets, S.; McCusker, L.B.; Davis, M.E. Synthesis and characterization of CIT-13, a germanosilicate molecular sieve with extra-large pore openings. Chem. Mater. 2016, 28, 6250–6259. [Google Scholar] [CrossRef]
- Chen, F.-J.; Gao, Z.-H.; Liang, L.-L.; Zhang, J.; Du, H.-B. Facile preparation of extra-large pore zeolite ITQ-37 based on supramolecular assemblies as structure-directing agents. CrystEngComm 2016, 18, 2735–2741. [Google Scholar] [CrossRef]
- Rojas, A.; Camblor, M.A. HPM-2, the layered precursor to zeolite MTF. Chem. Mater. 2014, 26, 1161–1169. [Google Scholar] [CrossRef]
- Mintova, S.; Barrier, N. Verified Synthesis of Zeolitic Materials, 3rd ed.; Elsevier (On behalf of Synthesis Commission of the International Zeolite Association): Amsterdam, The Netherlands, 2016. [Google Scholar]
- Tang, F.; Ohto, T.; Hasegawa, T.; Bonn, M.; Nagata, Y. [small pi]+-[small pi]+ stacking of imidazolium cations enhances molecular layering of room temperature ionic liquids at their interfaces. Phys. Chem. Chem. Phys. 2017, 19, 2850–2856. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.W.; Gómez-Hortigüela, L.; Rojas, A.; Pergher, S.B.C. Fluoride-mediated synthesis of TON and MFI zeolites using 1-butyl-3-methylimidazolium as structure-directing agent. Microporous Mesoporous Mater. 2017, 252, 29–36. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–701. [Google Scholar] [CrossRef] [PubMed]
- Camblor, M.A.; Bong Hong, S. Synthetic silicate zeolites: Diverse materials accessible through geoinspiration. In Porous Materials; John Wiley & Sons, Ltd.: New York, NY, USA, 2010; pp. 265–325. [Google Scholar]
- Lowenstein, W. The distribution of aluminium in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Baerlocher, C.; McCusker, L.B.; International Zeolite Association. Database of Zeolite Structures. 2007. Available online: http://www.iza-structure.org/databases/ (accessed on 22 July 2017).
- McCusker, L.B.; Liebau, F.; Engelhardt, G. Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts: (IUPAC recommendations 2001). Pure Appl. Chem. 2001, 73, 381–394. [Google Scholar] [CrossRef]
- McCusker, L.B.; Liebau, F.; Englehardt, G. Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts (IUPAC recommendations 2001): Physical chemistry division commision on colloid and surface chemistry including catalysis. Microporous Mesoporous Mater. 2003, 58, 3–13. [Google Scholar] [CrossRef]
- Martinez-Sánchez, P.C.; Perez-Pariente, J. Zeolites and Ordered Porous Solids: Fundamentals and Applications; Universitat Politècnica de València: València, Spain, 2011. [Google Scholar]
- Li, Y.; Yu, J. New stories of zeolite structures: Their descriptions, determinations, predictions, and evaluations. Chem. Rev. 2014, 114, 7268–7316. [Google Scholar] [CrossRef] [PubMed]
- Anurova, N.A.; Blatov, V.A.; Ilyushin, G.D.; Proserpio, D.M. Natural tilings for zeolite-type frameworks. J. Phys. Chem. C 2010, 114, 10160–10170. [Google Scholar] [CrossRef]
- Blatov, V.A.; Delgado-Friedrichs, O.; O’Keeffe, M.; Proserpio, D.M. Three-periodic nets and tilings: Natural tilings for nets. Acta Crystallogr. Sect. A 2007, 63, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Flanigen, E.M.; Lok, B.M.; Patton, R.L.; Wilson, S.T. Aluminophosphate molecular sieves and the periodic table. In Studies in Surface Science and Catalysis; Murakami, Y., Iijima, A.I., Ward, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 28, pp. 103–112. [Google Scholar]
- Pastore, H.O.; Coluccia, S.; Marchese, L. Porous aluminophosphates: From molecular sieves to designed acid catalysts. Ann. Rev. Mater. Res. 2005, 35, 351–395. [Google Scholar] [CrossRef]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate molecular sieves: A new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Yu, J.; Xu, R. Rich structure chemistry in the aluminophosphate family. Acc. Chem. Res. 2003, 36, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lombard, A.; Simon-Masseron, A.; Rouleau, L.; Cabiac, A.; Patarin, J. Synthesis and characterization of core/shell Al-ZSM-5/Silicalite-1 zeolite composites prepared in one step. Microporous Mesoporous Mater. 2010, 129, 220–227. [Google Scholar] [CrossRef]
- Abraham, A.; Lee, S.-H.; Shin, C.-H.; Bong Hong, S.; Prins, R.; van Bokhoven, J.A. Influence of framework silicon to aluminium ratio on aluminium coordination and distribution in zeolite beta investigated by 27Al MAS and 27Al MQ MAS NMR. Phys. Chem. Chem. Phys. 2004, 6, 3031–3036. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, E.J.; Lopez-Arbeloa, F.; Hong, S.B.; Camblor, M.A. Microporous aluminophosphates synthesized with 1,2,3-trimethylimidazolium and fluoride. Dalton Trans. 2016, 45, 7616–7626. [Google Scholar] [CrossRef] [PubMed]
- Barthomeuf, D. Topological model for the compared acidity of SAPOs and SiAl zeolites. Zeolites 1994, 14, 394–401. [Google Scholar] [CrossRef]
- Barthomeuf, D. Topology and maximum content of isolated species (Al, Ga, Fe, B, Si, ...) in a zeolitic framework. An approach to acid catalysis. J. Phys. Chem. 1993, 97, 10092–10096. [Google Scholar] [CrossRef]
- Vomscheid, R.; Briend, M.; Peltre, M.J.; Man, P.P.; Barthomeuf, D. The role of the template in directing the Si distribution in SAPO zeolites. J. Phys. Chem. 1994, 98, 9614–9618. [Google Scholar] [CrossRef]
- Yu, J.; Xu, R. Insight into the construction of open-framework aluminophosphates. Chem. Soc. Rev. 2006, 35, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Masters, A.F.; Maschmeyer, T. Zeolites—From curiosity to cornerstone. Microporous Mesoporous Mater. 2011, 142, 423–438. [Google Scholar] [CrossRef]
- Noorduin, W.L.; Vlieg, E.; Kellogg, R.M.; Kaptein, B. From Ostwald ripening to single chirality. Angew. Chem. Int. Ed. 2009, 48, 9600–9606. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; San-Roman, M.L.; Zicovich-Wilson, C.M.; Camblor, M.A. Host-guest stabilization of a zeolite strained framework: In situ transformation of zeolite MTW into the less dense and more strained ITW. Chem. Mater. 2013, 25, 729–738. [Google Scholar] [CrossRef]
- Davis, M.E.; Lobo, R.F. Zeolite and molecular sieve synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Lobo, R.F.; Zones, S.I.; Davis, M.E. Structure-direction in zeolite synthesis. J. Incl. Phenom. Mol. Recognit. Chem. 1995, 21, 47–78. [Google Scholar]
- Flanigen, E.M.; Bennett, J.M.; Grose, R.W.; Cohen, J.P.; Patton, R.L.; Kirchner, R.M.; Smith, J.V. Silicalite, a new hydrophobic crystalline silica molecular sieve. Nature 1978, 271, 512–516. [Google Scholar] [CrossRef]
- Zones, S.I.; Hwang, S.-J.; Elomari, S.; Ogino, I.; Davis, M.E.; Burton, A.W. The fluoride-based route to all-silica molecular sieves; a strategy for synthesis of new materials based upon close-packing of guest-host products. Comptes Rendus Chim. 2005, 8, 267–282. [Google Scholar] [CrossRef]
- Vidal-Moya, J.A.; Blasco, T.; Rey, F.; Corma, A.; Puche, M. Distribution of fluorine and germanium in a new zeolite structure ITQ-13 studied by 19F nuclear magnetic resonance. Chem. Mater. 2003, 15, 3961–3963. [Google Scholar] [CrossRef]
- Moteki, T.; Lobo, R.F. A general method for aluminum incorporation into high-silica zeolites prepared in fluoride media. Chem. Mater. 2016, 28, 638–649. [Google Scholar] [CrossRef]
- Moliner, M.; Rey, F.; Corma, A. Towards the rational design of efficient organic structure-directing agents for zeolite synthesis. Angew. Chem. Int. Ed. 2013, 52, 13880–13889. [Google Scholar] [CrossRef] [PubMed]
- Koller, H.; Wölker, A.; Villaescusa, L.A.; Díaz-Cabañas, M.J.; Valencia, S.; Camblor, M.A. Five-coordinate silicon in high-silica zeolites. J. Am. Chem. Soc. 1999, 121, 3368–3376. [Google Scholar] [CrossRef]
- Tian, P.; Su, X.; Wang, Y.; Xia, Q.; Zhang, Y.; Fan, D.; Meng, S.; Liu, Z. Phase-transformation synthesis of SAPO-34 and a novel SAPO molecular sieve with RHO framework type from a SAPO-5 precursor. Chem. Mater. 2011, 23, 1406–1413. [Google Scholar] [CrossRef]
- Althoff, R.; Unger, K.; Schüth, F. Is the formation of a zeolite from a dry powder via a gas phase transport process possible? Microporous Mater. 1994, 2, 563–564. [Google Scholar] [CrossRef]
- Morris, R.E.; James, S.L. Solventless synthesis of zeolites. Angew. Chem. Int. Ed. 2013, 52, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Weigel, S.J. The synthesis of molecular sieves from non-aqueous solvents. Chem. Soc. Rev. 1997, 26, 309–317. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. 2.4-structure of five-membered rings with two or more heteroatoms. In Handbook of Heterocyclic Chemistry, 3th ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 139–209. [Google Scholar]
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. 3.4-reactivity of five-membered rings with two or more heteroatoms. In Handbook of Heterocyclic Chemistry, 3th ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 473–604. [Google Scholar]
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. 4.3-synthesis of monocyclic rings with two or more heteroatoms. In Handbook of Heterocyclic Chemistry, 3th ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 704–796. [Google Scholar]
- Dong, L.; Zheng, D.X.; Wei, Z.; Wu, X.H. Synthesis of 1,3-dimethylimidazolium chloride and volumetric property investigations of its aqueous solution. Int. J. Thermophys. 2009, 30, 1480. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. 1,2-azoles and 1,3-azoles. In Heterocyclic Chemistry at a Glance; John Wiley & Sons, Ltd.: New York, NY, USA, 2012; pp. 107–121. [Google Scholar]
- Chemaxon. Marvinsketch, 6; Chemaxon: Budapest, Hungary, 2013. [Google Scholar]
- Katritzky, A.; Ramsden, C.; Joule, J.; Zhdankin, V. Handbook of Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Morris, R.E. Ionic liquids and microwaves—Making zeolites for emerging applications. Angew. Chem. Int. Ed. 2008, 47, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E. Ionothermal synthesis-ionic liquids as functional solvents in the preparation of crystalline materials. Chem. Commun. 2009, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Arteaga, O.; Kahr, B.; Camblor, M.A. Synthesis, structure, and optical activity of HPM-1, a pure silica chiral zeolite. J. Am. Chem. Soc. 2013, 135, 11975–11984. [Google Scholar] [CrossRef] [PubMed]
- Mignoni, M.L.; de Souza, M.O.; Pergher, S.B.C.; de Souza, R.F.; Bernardo-Gusmão, K. Nickel oligomerization catalysts heterogenized on zeolites obtained using ionic liquids as templates. Appl. Catal. A Gen. 2010, 374, 26–30. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Pimentel, B.R.; Parulkar, A.; Zhou, E.-K.; Brunelli, N.A.; Lively, R.P. Zeolitic imidazolate frameworks: Next-generation materials for energy-efficient gas separations. ChemSusChem 2014, 7, 3202–3240. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal-organic frameworks as platforms for functional materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting. Adv. Sci. 2017, 4, 1600371. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2008. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2004. [Google Scholar]
- Yu, J.; Williams, I.D. Two unusual layer aluminophosphates templated by imidazolium ions; [N2C3H5][AlP2O8H2·2H2O] and 2[N2C3H5][Al3P4O16H]. J. Solid State Chem. 1998, 136, 141–144. [Google Scholar] [CrossRef]
- Fayad, E.J.; Bats, N.; Kirschhock, C.E.A.; Rebours, B.; Quoineaud, A.-A.; Martens, J.A. A rational approach to the ionothermal synthesis of an AlPO4 molecular sieve with an LTA-type framework. Angew. Chem. Int. Ed. 2010, 49, 4585–4588. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Gomez-Hortiguela, L.; Camblor, M.A. Benzylimidazolium cations as zeolite structure-directing agents. Differences in performance brought about by a small change in size. Dalton Trans. 2013, 42, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-H.; Chen, F.-J.; Xu, L.; Sun, L.; Xu, Y.; Du, H.-B. A stable extra-large-pore zeolite with intersecting 14- and 10-membered-ring channels. Chem. A Eur. J. 2016, 22, 14367–14372. [Google Scholar] [CrossRef] [PubMed]
- Archer, R.H.; Zones, S.I.; Davis, M.E. Imidazolium structure directing agents in zeolite synthesis: Exploring guest/host relationships in the synthesis of SSZ-70. Microporous Mesoporous Mater. 2010, 130, 255–265. [Google Scholar] [CrossRef]
- Xu, R.; Chen, J.; Gao, Z.; Yan, W. From Zeolites to Porous MOF Materials-the 40th Anniversary of International Zeolite Conference; Elsevier Science: Beijing, China, 2007. [Google Scholar]
- Martínez-Blanes, J.M.; Szyja, B.M.; Romero-Sarria, F.; Centeno, M.Á.; Hensen, E.J.M.; Odriozola, J.A.; Ivanova, S. Multiple zeolite structures from one ionic liquid template. Chem. A Eur. J. 2013, 19, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; He, D.; Xu, S.; Song, Z.; Zhang, M.; Wei, Y.; He, Y.; Xu, S.; Liu, Z.; Xu, Y. Imidazolium-based ionic liquids as novel organic SDA to synthesize high-silica Y zeolite. Microporous Mesoporous Mater. 2015, 204, 1–7. [Google Scholar] [CrossRef]
- Ma, H.; Xu, R.; You, W.; Wen, G.; Wang, S.; Xu, Y.; Wang, B.; Wang, L.; Wei, Y.; Xu, Y.; et al. Ionothermal synthesis of gallophosphate molecular sieves in 1-alkyl-3-methyl imidazolium bromide ionic liquids. Microporous Mesoporous Mater. 2009, 120, 278–284. [Google Scholar] [CrossRef]
- Parodia, A.; Emmerich, D.; Bernardo-Gusmao, K.; Mignoni, M.L. Estudo da síntese da zeólita tipo TON utilizando líquidos iônicos como direcionadores de estrutura. Perspectiva (Erexim) 2014, 38, 61–69. [Google Scholar]
- Dodin, M.; Paillaud, J.-L.; Lorgouilloux, Y.; Caullet, P.; Elkaïm, E.; Bats, N. A zeolitic material with a three-dimensional pore system formed by straight 12- and 10-ring channels synthesized with an imidazolium derivative as structure-directing agent. J. Am. Chem. Soc. 2010, 132, 10221–10223. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Martínez-Morales, E.; Zicovich-Wilson, C.M.; Camblor, M.A. Zeolite synthesis in fluoride media: Structure direction toward ITW by small methylimidazolium cations. J. Am. Chem. Soc. 2012, 134, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.E.; Deimund, M.A.; Xie, D.; Davis, M.E. Synthesis of RTH-type zeolites using a diverse library of imidazolium cations. Chem. Mater. 2015, 27, 3756–3762. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. The ionothermal synthesis of cobalt aluminophosphate zeolite frameworks. J. Am. Chem. Soc. 2006, 128, 2204–2205. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Chen, L.; Zhang, Y.; Yuan, Y.; Xu, L.; Zhang, X.; Xu, L. Rapid synthesis of ZSM-22 zeolites using imidazolium-based ionic liquids as osdas in fluoride media. Microporous Mesoporous Mater. 2016, 236, 193–201. [Google Scholar] [CrossRef]
- Lorgouilloux, Y.; Dodin, M.; Paillaud, J.-L.; Caullet, P.; Michelin, L.; Josien, L.; Ersen, O.; Bats, N. IM-16: A new microporous germanosilicate with a novel framework topology containing d4r and mtw composite building units. J. Solid State Chem. 2009, 182, 622–629. [Google Scholar] [CrossRef]
- Zones, S.I.; Burton, A.W. Diquaternary structure-directing agents built upon charged imidazolium ring centers and their use in synthesis of one-dimensional pore zeolites. J. Mater. Chem. 2005, 15, 4215–4223. [Google Scholar] [CrossRef]
- Kore, R.; Srivastava, R. Synthesis of triethoxysilane imidazolium based ionic liquids and their application in the preparation of mesoporous ZSM-5. Catal. Commun. 2012, 18, 11–15. [Google Scholar] [CrossRef]
- Boal, B.W.; Deem, M.W.; Xie, D.; Kang, J.H.; Davis, M.E.; Zones, S.I. Synthesis of germanosilicate molecular sieves from mono- and di-quaternary ammonium osdas constructed from benzyl imidazolium derivatives: Stabilization of large micropore volumes including new molecular sieve CIT-13. Chem. Mater. 2016, 28, 2158–2164. [Google Scholar] [CrossRef]
- Khoo, D.Y.; Awala, H.; Mintova, S.; Ng, E.-P. Synthesis of AlPO-5 with diol-substituted imidazolium-based organic template. Microporous Mesoporous Mater. 2014, 194, 200–207. [Google Scholar] [CrossRef]
- Ng, E.-P.; Sekhon, S.S.; Mintova, S. Discrete MnAlPO-5 nanocrystals synthesized by an ionothermal approach. Chem. Commun. 2009, 0, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Variani, Y.M.; Rojas, A.; Gomez-Hortiguela, L.; Pergher, S.B.C. Study of the performance of imidazolium-derived cations as structure directing agents in the synthesis of zeolites in fluoride media. New J. Chem. 2016, 40, 7968–7977. [Google Scholar] [CrossRef]
- Pinar, A.B.; McCusker, L.B.; Baerlocher, C.; Schmidt, J.; Hwang, S.J.; Davis, M.E.; Zones, S.I. Location of ge and extra-framework species in the zeolite ITQ-24. Dalton Trans. 2015, 44, 6288–6295. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.E.; Xie, D.; Rea, T.; Davis, M.E. CIT-7, a crystalline, molecular sieve with pores bounded by 8 and 10-membered rings. Chem. Sci. 2015, 6, 1728–1734. [Google Scholar] [CrossRef]
- Wen, H.; Zhou, Y.; Xie, J.; Long, Z.; Zhang, W.; Wang, J. Pure-silica ZSM-22 zeolite rapidly synthesized by novel ionic liquid-directed dry-gel conversion. RSC Adv. 2014, 4, 49647–49654. [Google Scholar] [CrossRef]
- Bernardo-Maestro, B.; López-Arbeloa, F.; Pérez-Pariente, J.; Gómez-Hortigüela, L. Supramolecular chemistry controlled by conformational space during structure direction of nanoporous materials: Self-assembly of ephedrine and pseudoephedrine. J. Phys. Chem. C 2015, 119, 28214–28225. [Google Scholar] [CrossRef]
- Kubota, Y.; Helmkamp, M.M.; Zones, S.I.; Davis, M.E. Properties of organic cations that lead to the structure-direction of high-silica molecular sieves. Microporous Mater. 1996, 6, 213–229. [Google Scholar] [CrossRef]




| Pore Size | Number of Tetrahedra (MR 1) | Pore Diameter (Å) | Example |
|---|---|---|---|
| Small | 8 | 4 | PST-1 (NAT) |
| Medium | 10 | 5.5 | ZSM-5 (MFI) |
| Large | 12 | 7.5 | ZSM-12 (MTW) |
| Extra-large | >12 | >7.5 | CIT-5 (CFI) |
| Observed Bonds | Impossible Bonds |
|---|---|
| Al-O-P | P-O-P |
| Si-O-Si | P-O-Si |
| Si-O-Al | Al-O-Al |
| M-O-P | M-O-Al |
| M-O-P-O-M | M-O-M |
| Cation | Type | |
|---|---|---|
| Neutral compound | ||
| Imidazole |  | [ImidH]AlP2O8H2·2H2O, [ImidH]Al3P4O16H [63] |
| Bi-substituted imidazolium | ||
| 1-Benzyl-3-methylimidazolium |  | AFI, LTA [64], MFI, MTW [65] |
| 3-Methyl-1-[(2-methylphenyl) methyl]-1H-imidazol-3-ium |  | NUD-2 ¥ [66] |
| 3-Methyl-1-[(3-methylphenyl) methyl]-1H-imidazol-3-ium |  | CIT-13 ¥, MFI [3], NUD-2 ¥ [66] |
| 1-[(3,5-Dimethylphenyl)methyl]-3-methyl-1H-imidazol-3-ium |  | CIT-13 ¥ [3] |
| 1,3-Bis(1-adamantyl) imidazolium |  | AFX, CFI, FAU, MOR, STF [67] |
| 1,3-Bis(bicyclo[2.2.1]heptan-2-yl)imidazolium |  | BEA*, SSZ-70 ¥ [67] |
| 1,3-Bis(cycloheptyl) imidazolium |  | BEA*, MOR, SSZ-70 ¥ [67] |
| 1,3-Bis(cyclohexyl)imidazolium |  | BEA*, EUO, FAU, MOR, SSZ-70 ¥ [67] |
| 1,3-Bis(cyclohexylmethyl) imidazolium |  | BEA*, FAU, MOR, MTW, SSZ-70 ¥ [67] |
| 1,3-Bis(cyclopentyl) imidazolium |  | BEA*, SSZ-70 ¥ [67] |
| 1,3-Bis(cyclooctyl)imidazolium |  | BEA*, FAU, MOR [67] |
| 1,3-Bis(pentan-3-yl)imidazolium |  | FAU, MTW, MOR, SSZ-70 ¥ [67] |
| 1,3-Bis(tert-butyl)imidazolium |  | FAU, MOR [67] |
| 1,3-Bis(2,4,4-trimethylpentan-2-yl)imidazolium |  | FAU [67] |
| 1-Butyl-3-methylimidazolium |  | AEL [68], AFI [64], ANA, BEA*, MFI [8,56,69], FAU [70], LTA [71], TON [8,72], UWY $ [73] |
| 1,3-Dimethylimidazolium |  | ITW $ [74], FER [75], TON [67] |
| 1,3-Diethylimidazolium |  | MFI, TON [67] |
| 1,3-Diisopropylimidazolium |  | MTT, MTW, SSZ-70 ¥ [67] |
| 1,3-Diisobutylimidazolium |  | BEA*, FAU, MOR, MTW, SSZ-70 ¥ [67] |
| 1-Ethyl-3-methylimidazolium |  | AEI, SIV $, SOD [76], AEL, CHA, LTA [64], CLO [71], TON [77], UOS $ [78] |
| 1-Hexyl-3-methylimidazolium |  | LTA [71] |
| 1-Isopropyl-3-methyl imidazolium |  | MRE*, MTT [79] |
| 1-Methyl-3-pentylimidazolium |  | LTA [71] |
| 1-Methyl-3-propylimidazolium |  | LTA [71] |
| 1-(Propan-2-yl)-3-(4-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl]butyl)-1H-imidazol-3-ium |  | MTW, TON [79] |
| 1-(Propan-2-yl)-3-(5-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] pentyl)-1H-imidazol-3-ium |  | EUO, MFI, MRE* [79] |
| 1-(Propan-2-yl)-3-(6-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] hexyl)-1H-imidazol-3-ium |  | EUO, MFI, MRE*, MTW, TON [79] |
| 1-(Propan-2-yl)-3-(7-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] heptyl)-1H-imidazol-3-ium |  | MTT [79] |
| 1-(Propan-2-yl)-3-(8-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] octyl)-1H-imidazol-3-ium |  | MRE*, MTT, MTW [79] |
| 1-(Propan-2-yl)-3-(9-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] nonyl)-1H-imidazol-3-ium |  | MFI, MRE*, MTW [79] |
| 1-(Propan-2-yl)-3-(10-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] decyl)-1H-imidazol-3-ium |  | MFI, MRE*, MTW, NES, TON [79] |
| 1-(Propan-2-yl)-3-(11-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] undecyl)-1H-imidazol-3-ium |  | MTT [79] |
| 1-(Propan-2-yl)-3-(12-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] dodecyl)-1H-imidazol-3-ium |  | MRE*, MTT [79] |
| 1-(Propan-2-yl)-3-(14-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] tetradecyl)-1H-imidazol-3-ium |  | FER, MTW [79] |
| 1-(Propan-2-yl)-3-(16-[1-(propan-2-yl)-1H-imidazol-3-ium-3-yl] hexadecyl)-1H-imidazol-3-ium |  | FER [79] |
| 1-(Triethoxysilyl propyl)-3-dodecylimidazolium |  | MFI [80] |
| 1-(Triethoxysilyl propyl)-3-methylimidazolium |  | MFI [80] |
| Tri-substituted imidazolium | ||
| 1-Benzyl-2,3-dimethyl imidazolium |  | ITW $, MTW [65] |
| 2,3-Dimethyl-1-[(2-methyl phenyl)methyl]-1H-imidazol-3-ium |  | CIT-13 ¥, IWS $, LTA [81] |
| 2,3-Dimethyl-1-[(3-methyl phenyl)methyl]-1H-imidazol-3-ium |  | CIT-13 ¥ [3] |
| 2,3-Dimethyl-1-[(4-methyl phenyl)methyl]-1H-imidazol-3-ium |  | BEC, LTA [81] |
| 1-[(3,5-Dimethylphenyl) methyl]-2,3-dimethyl-1H-imidazol-3-ium |  | CIT-13 ¥ [3] |
| 1-((2-[(1,2-Dimethyl-1H-imidazol-3-ium-3-yl)methyl]phenyl)-methyl)-2,3-dimethyl-1H-imidazol-3-ium |  | BEA* [81] |
| 1-((3-[(1,2-Dimethyl-1H-imidazol-3-ium-3-yl)methyl]phenyl)-methyl)-2,3-dimethyl-1H-imidazol-3-ium |  | BEA*, IWS $ [81] |
| 1-((4-[(1,2-Dimethyl-1H-imidazol-3-ium-3-yl)methyl]phenyl)methyl)-2,3-dimethyl-1H-imidazol-3-ium |  | BEA*, BEC [81] |
| 1-(2,3-Dihydroxipropyl)-2,3-dimethylimidazolium |  | AFI [82] |
| 2-Ethyl-1,3-dimethyl imidazolium |  | CSV $, ITW $, MTW, RTH, STF, STW$ [75] |
| 1-Ethyl-2,3-dimethylimidazolium |  | AFI [83], ITW $, MTW [32] |
| 2-Isopropyl-1,3-dimethylimidazolium |  | MOR, STF [75] |
| 1,1′,1′′-(2,4,6-Trimethyl benzene-1,3,5-triyl)tris (methylene)tris-(3-methyl-1H-imidazol-3-ium) |  | -ITV $ [4] |
| 1,2,3-Trimethylimidazolium |  | AFI, CHA, HPM-3 ¥ [25], ITW $ [78], RTH [75], MFI, STF [84] |
| 1,3,4-Trimethylimidazolium |  | ITW $, TON [74], MOR, RTH [75] |
| 1,3,5-Tris(1,2-dimethyl imidazolium)benzene |  | IWR $, LTA [85] |
| Tetra-substituted imidazolium | ||
| 2-Ethyl-1,3,4-trimethyl imidazolium |  | ITW $, MTF, STW $ [5,55], MOR, MTW, RTH [75] |
| 1,2,3,4-Tetramethyl imidazolium |  | MOR, RTH, STW $ [75] |
| 1,2,3-Triethyl-4-methylimidazolium |  | STF [84] |
| Penta-substituted imidazolium | ||
| 1,2,4,5-Tetramethyl-3-[3-(tetramethyl-1H-imidazol-3-ium-3-yl)propyl]-1H-imidazol-3-ium |  | CSV $, IWV $, STW $ [86] |
| 1,2,3,4,5-Pentamethyl imidazolium |  | RTH, STW $ [75] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinaches, P.; Bernardo-Gusmão, K.; Pergher, S.B.C. An Introduction to Zeolite Synthesis Using Imidazolium-Based Cations as Organic Structure-Directing Agents. Molecules 2017, 22, 1307. https://doi.org/10.3390/molecules22081307
Vinaches P, Bernardo-Gusmão K, Pergher SBC. An Introduction to Zeolite Synthesis Using Imidazolium-Based Cations as Organic Structure-Directing Agents. Molecules. 2017; 22(8):1307. https://doi.org/10.3390/molecules22081307
Chicago/Turabian StyleVinaches, Paloma, Katia Bernardo-Gusmão, and Sibele B. C. Pergher. 2017. "An Introduction to Zeolite Synthesis Using Imidazolium-Based Cations as Organic Structure-Directing Agents" Molecules 22, no. 8: 1307. https://doi.org/10.3390/molecules22081307
APA StyleVinaches, P., Bernardo-Gusmão, K., & Pergher, S. B. C. (2017). An Introduction to Zeolite Synthesis Using Imidazolium-Based Cations as Organic Structure-Directing Agents. Molecules, 22(8), 1307. https://doi.org/10.3390/molecules22081307