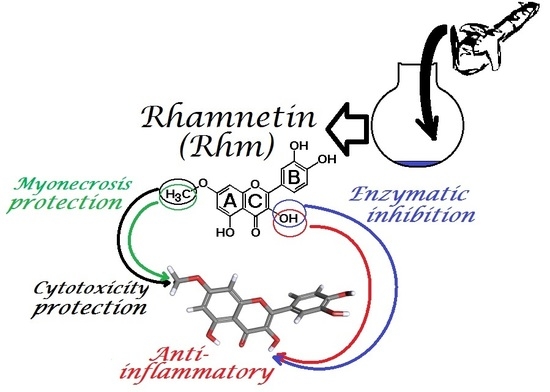
Evaluation of Rhamnetin as an Inhibitor of the Pharmacological Effect of Secretory Phospholipase A2
Abstract
:1. Introduction
2. Results
2.1. Purity of sPLA2
2.2. Enzymatic, Circular Dichroism, Edema, and Myotoxic and Cytotoxic Effects
2.2.1. Enzymatic Assay
2.2.2. Circular Dichroism
2.2.3. Paw Edema Assay of Chemically-Treated sPLA2
2.2.4. CK Levels of Chemically-Treated sPLA2
2.2.5. Cytotoxic Assay of Methylated Quercetins
2.3. Edema Assay After Injection of sPLA2
3. Discussion
4. Materials and Methods
4.1. Flavonoids and Reagents
4.2. Isolation, Enzymatic Kinetics, and Spectroscopic Evaluation of sPLA2
4.2.1. Purification of sPLA2 from Bothrops jararacussu
4.2.2. Enzymatic Characterization of sPLA2
4.2.3. Circular Dichroism (CD)
4.3. Pharmacological Assay
4.3.1. Paw Edema Evaluation
4.3.2. CK Level Measurement
4.4. Cytotoxic Effects
4.5. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| sPLA2 | Secretory phospholipase A2 |
| Q | Quercetin |
| 3MQ | 3-O-Methylquercetin |
| Rhm | Rhamnetin |
| Rhz | Rhamnazin |
References
- Oliveira, S.C.B.; Fonseca, F.V.; Antunes, E.; Camargo, E.A.; Morganti, R.P.; Aparício, R.; Toyama, D.O.; Beriam, L.O.S.; Nunes, E.V.; Cavada, B.S.; et al. Modulation of the pharmacological effects of enzymatically-active PLA2 by BTL-2, an isolectin isolated from the Bryothamnion triquetrum red alga. BMC Biochem. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.A.; de Oliveira, S.C.B.; Diz Filho, E.B.S.; Fonseca, V.F.; Baldissera, L., Jr.; Antunes, E.; Ximenes, R.M.; Moteiro, H.S.A.; Rabello, M.M.; Hernandes, M.Z.; et al. Quercetin as an inhibitor of snake venom secretory phospholipase A2. Chem. Biol. Interact. 2011, 189, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J.; Haapamaki, M.M.; Gronroos, J.M. Roles of secretory phospholipases A2 in inflammatory diseases and trauma. BBA Mol. Cell Biol. Lipids 2000, 1488, 83–90. [Google Scholar] [CrossRef]
- Ximenes, R.M.; Rabello, M.M.; Araújo, R.M.; Silveira, E.R.; Fagundes, F.H.R.; Diz-Filho, E.B.S.; Buzzo, S.C.; Soares, V.C.G.; de O. Toyama, D.; Gaeta, H.H.; et al. Inhibition of neurotoxic secretory phospholipases A2 enzymatic, edematogenic, and myotoxic activities by harpalycin 2, an isoflavone isolated from Harpalyce brasiliana benth. Evid. Based Complement. Alternat. Med. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Toyama, D.O.; Gaeta, H.H.; de Pinho, M.V.; Ferreira, M.J.; Romoff, P.; Matioli, F.F.; Magro, A.J.; Fontes, M.R.; Toyama, M.H. An evaluation of 3-rhamnosylquercetin, a glycosylated form of quercetin, against the myotoxic and edematogenic effects of sPLA 2 from Crotalus durissus terrificus. Biomed. Res. Int. 2014, 2014, 1–11. [Google Scholar]
- Toyama, D.O.; Ferreira, M.J.; Romoff, P.; Fávero, O.A.; Gaeta, H.H.; Toyama, M.H. Effect of chlorogenic acid (5-Caffeoylquinic Acid) isolated from Baccharis oxyodonta on the structure and pharmacological activities of secretory phospholipase A2 from Crotalus durissus terrificus. Biomed. Res. Int. 2014, 2014, 1–10. [Google Scholar]
- Behling, E.B.; Sendão, M.C.; Francescato, H.D.C.; Antunes, L.M.G.; Bianchi, M.L.P. Flavonoid quercetin: General aspects and biologicals actions. Alim. Nutr. 2004, 15, 285–292. [Google Scholar]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily Quercetin Supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [PubMed]
- Morikawa, K.; Nonak, M.; Narahara, M.; Torii, I.; Kawaguchi, K.; Yoshikawa, T.; Kumazawa, Y.; Morikawa, S. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci. J. 2003, 74, 709–721. [Google Scholar] [CrossRef]
- Souza, K.C.B.; Schapoval, E.E.S.; Bassani, V.L. LC determination of flavonoids: Separation of quercetin, luteolin and 3-O-methylquercetin in Achyrocline satureioides preparations. J. Pharm. Biomed. Anal. 2002, 28, 771–777. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Lee, E.; Jeong, K.W.; Shin, A.; Heo, Y.S.; Kim, Y. Anti-inflammatory activity of rhamnetin and a model of its binding to c-jun NH2-terminal kinase 1 and p38 MAPK. J. Nat. Prod. 2014, 77, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ertan, M.; Seligmann, O. Rhamnazin-und Rhamnetin-3-O-Trioside aus Rhamnus petiolaris. Phytochemistry 1973, 13, 857–860. [Google Scholar] [CrossRef]
- Kuhnle, G.; Spencer, J.P.; Schroete, H.; Shenoy, B.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C.; Hahn, U. Epicatechin and catechin are Omethylated and glucuronidated in the small intestine. Biochem. Biophys. Res. Commun. 2000, 277, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Núñez, J.; Cintra, A.C.O.; Homsi-Brandeburgo, M.I.; Giglio, J.R. Skeletal muscle degeneration and regeneration after injection of bothropstoxin-II, a phospholipase A2 isolated from the venom of the snake Bothrops jararacussu. Exp. Mol. Pathol. 1991, 55, 217–229. [Google Scholar] [CrossRef]
- Borges, M.H.; Soares, A.M.; Rodrigues, V.M.; Andrião-Escarso, S.H.; Diniz, H.; Hamaguchi, A.; Quintero, A.; Lizano, S.; Gutiérrez, J.M.; Giglio, J.R.; Homsi-Brandeburgo, M.I. Effects of aqueous extract of Casearia sylvestris (Flacourtiaceae) on actions of snake and bee venoms and on activity of phospholipases A2. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 127, 21–30. [Google Scholar] [CrossRef]
- Núñes, V.; Castro, V.; Murillo, R.; Ponce-Soto, L.A.; Merfort, I.; Lomonte, B. Nhibitory effects of Piper umbellatum and Piper peltatum extracts towards myotoxic phospholipases A2 from Bothrops snake venoms: Isolation of 4-nerolidylcatechol as active principle. Phytochemistry 2005, 66, 1017–1025. [Google Scholar]
- Vishnawath, B.S.; Fawzy, A.A.; Franson, R.C. Edema-inducing activity of phospholipase A2 purified from human synovial fluid and inhibition by aristolochic acid. Inflammation 1988, 12, 549–561. [Google Scholar] [CrossRef]
- Vishnawath, B.S.; Gowda, V.T. Interaction of aristolochic acid with Vipera russelli phospholipase A2: Its effect on enzymatic and pathologic activities. Toxicon 1987, 25, 927–937. [Google Scholar] [CrossRef]
- Vishnawath, B.S.; Kini, R.M.; Gowda, T.V. Characterization of three edema-inducing phospholipase A2 enzymes from Habu (Trimesurus flavoviridis) venom and their interaction with the alkaloid aristolochic acid. Toxicon 1987, 25, 501–515. [Google Scholar] [CrossRef]
- Vishnawath, B.S.; Rao, A.G.A.; Gowda, T.V. Interaction of phospholipase A2 from Vipera russeli venom with aristolochic acid: A circular dichroism study. Toxicon 1987, 25, 939–946. [Google Scholar] [CrossRef]
- Caoab, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Liu, L.; Guo, W.; Meydani, M. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J. Nutr. Biochem. 2006, 17, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Moalin, M.; Strijdonck, G.P.; Beckers, M.; Hagemen, G.; Borm, P.; Bast, A.; Haenen, G.R. A planar conformation and the hydroxyl groups in the B and C rings play a pivotal role in the antioxidant capacity of quercetin and quercetin derivatives. Molecules 2011, 16, 9636–9650. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.X. Adipocytes, Macrophages and Inflammation. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2014. [Google Scholar]
- Camargo, E.A.; Santana, D.G.; Silva, C.I.; Teixeira, S.A.; Toyama, M.H.; Cotrim, C.; Landucci, E.C.; Antunes, E.; Muscara, M.N.; Costa, S.K. Inhibition of inducible nitric oxide synthase-derived nitric oxide as a therapeutical target for acute pancreatitis induced by secretory phospholipase A2. Eur. J. Pain. 2014, 18, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? J. Immunol. Res. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- Zhang, W.; Li, B.; Guo, Y.; Bai, Y.; Wang, T.; Fu, K.; Sun, G. Rhamnetin attenuates cognitive deficit and inhibits hippocampal inflammatory response and oxidative stress in rats with traumatic brain injury. Cent. Eur. J. Immunol. 2015, 40, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. II Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Ramma-Luximon, A.; Aruoma, O.I. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fund. Mol. Mech. Mut. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.S.; Shih, C.M.; Wang, S.H.; Chen, T.T.; Lin, C.N.; Ko, W.C. Mechanisms of suppression of nitric oxide production by 3-O-methylquercetin in RAW 264.7 cells. J. Ethnopharmacol. 2006, 103, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Rajalingam, D.; Maity, T.K. Anti-inflammatory effect of O-methylated flavonol 2-(3,4-dihydroxy-phenyl)-3,5-dihydroxy-7-methoxy-chromen-4-one obtained from Cassia sophera Linn in rats. J. Ethnopharmacol. 2013, 147, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Ozipek, M.; Çalis, I.; Ertant, M.; Ruedi, P. Rhamnetin 3-p-coumaroylrhamninoside from Rhamnus petiolaris. Phytochemistry 1994, 37, 249–253. [Google Scholar] [CrossRef]
- Wei, B.L.; Lu, C.M.; Tsao, L.T.; Wang, J.P.; Lin, C.N. In vitro anti-inflammatory effects of quercetin 3-O-methyl ether and other constituents from Rhamnus species. Planta Med. 2001, 67, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Moalin, M. Quercetin and its Methylated Metabolites: The Chemical Basis of Activity. Ph.D. Thesis, Maastricht University, Maastricht, Netherlands, 2014. [Google Scholar]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Schluesener, H.J.; Meyermann, R.; Serhan, C.N. COX-3 the enzyme and the concept: Steps towards highly specialized pathways and precision therapeutics? Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 339–343. [Google Scholar] [CrossRef]
- Font-Nieves, M.; Sans-Fons, M.G.; Gorina, R.; Bonfill-Teixidor, E.; Salas-Pérdomo, A.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Induction of COX-2 Enzyme and Down-regulation of COX-1 Expression by Lipopolysaccharide (LPS) Control Prostaglandin E2 Production in Astrocytes. J. Biol. Chem. 2012, 287, 6454–6468. [Google Scholar] [CrossRef] [PubMed]
- Nattras, C.; Horwell, C.J.; Damby, D.E.; Brown, D.; Stone, V. The effect of aluminium and sodium impurities on the in vitro toxicity and pro-inflammatory potential of cristobalite. Environ. Res. 2017, 159, 164–175. [Google Scholar]
- Leslie, L.J.; Vasanthi, B.P.; Jackson, P.; Mabiala, M.M.J.A.; Pallet, R.; Stillman, C.J.P.; Marshall, L.J. A comparative study of electronic cigarette vapor extracts on airway-related cell lines in vitro. Inhal. Toxicol. 2017, 29, 126–136. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds sPLA2, 3MQ, Rhz, Rhm and Q are available from the authors. |



| sPLA2 | 3MQ | Rhm | Rhz | Q | |
|---|---|---|---|---|---|
| α-helix | 45.30% | 26.80% | 27.00% | 54.70% | 23.40% |
| β-sheet | 14.80% | 18.30% | 18.00% | 13.40% | 18.80% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novo Belchor, M.; Hessel Gaeta, H.; Fabri Bittencourt Rodrigues, C.; Ramos da Cruz Costa, C.; De Oliveira Toyama, D.; Domingues Passero, L.F.; Dalastra Laurenti, M.; Hikari Toyama, M. Evaluation of Rhamnetin as an Inhibitor of the Pharmacological Effect of Secretory Phospholipase A2. Molecules 2017, 22, 1441. https://doi.org/10.3390/molecules22091441
Novo Belchor M, Hessel Gaeta H, Fabri Bittencourt Rodrigues C, Ramos da Cruz Costa C, De Oliveira Toyama D, Domingues Passero LF, Dalastra Laurenti M, Hikari Toyama M. Evaluation of Rhamnetin as an Inhibitor of the Pharmacological Effect of Secretory Phospholipase A2. Molecules. 2017; 22(9):1441. https://doi.org/10.3390/molecules22091441
Chicago/Turabian StyleNovo Belchor, Mariana, Henrique Hessel Gaeta, Caroline Fabri Bittencourt Rodrigues, Caroline Ramos da Cruz Costa, Daniela De Oliveira Toyama, Luiz Felipe Domingues Passero, Marcia Dalastra Laurenti, and Marcos Hikari Toyama. 2017. "Evaluation of Rhamnetin as an Inhibitor of the Pharmacological Effect of Secretory Phospholipase A2" Molecules 22, no. 9: 1441. https://doi.org/10.3390/molecules22091441
APA StyleNovo Belchor, M., Hessel Gaeta, H., Fabri Bittencourt Rodrigues, C., Ramos da Cruz Costa, C., De Oliveira Toyama, D., Domingues Passero, L. F., Dalastra Laurenti, M., & Hikari Toyama, M. (2017). Evaluation of Rhamnetin as an Inhibitor of the Pharmacological Effect of Secretory Phospholipase A2. Molecules, 22(9), 1441. https://doi.org/10.3390/molecules22091441