Cyclodextrin-Catalyzed Organic Synthesis: Reactions, Mechanisms, and Applications
Abstract
:1. Introduction
2. Application of Cyclodextrin in Conventional Reaction
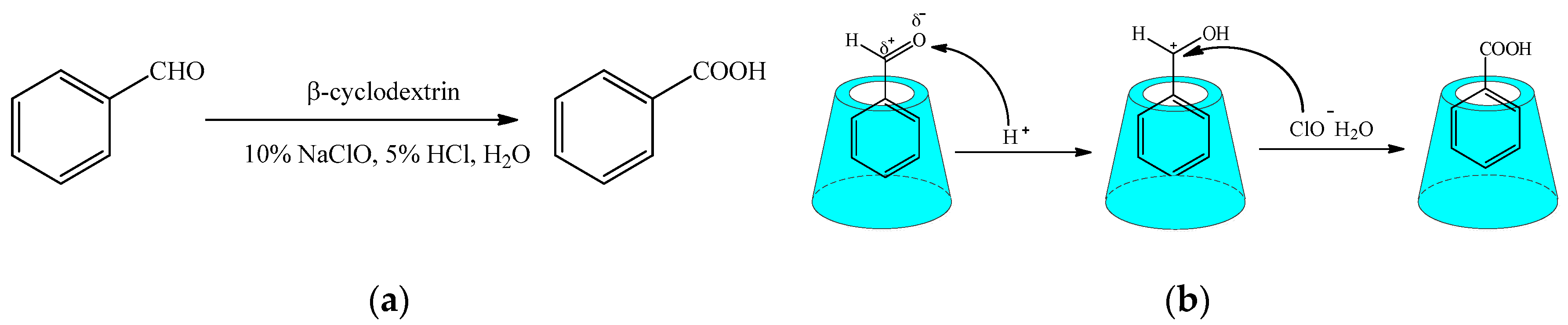
2.1. Conventional Oxidation Reaction
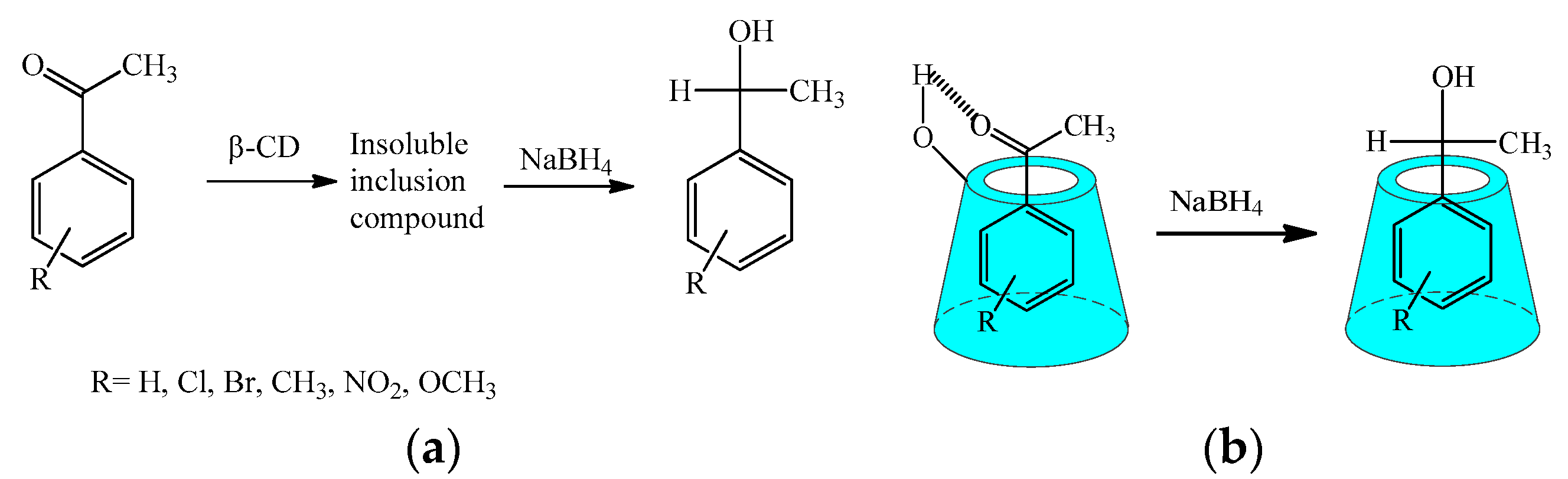
2.2. Conventional Reduction Reaction
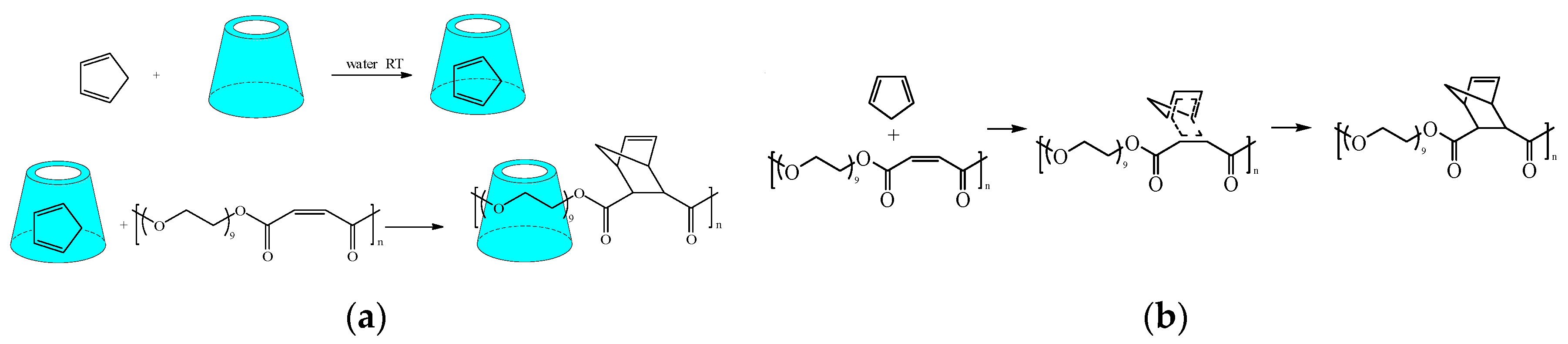
2.3. Conventional Addition Reaction
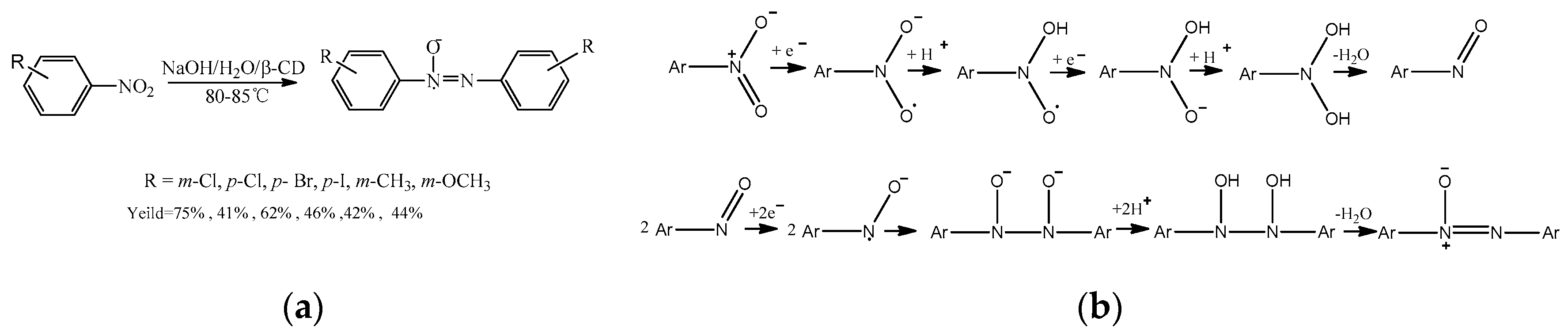
2.4. Conventional Substitution Reaction
2.5. Conventional Hydrolysis Reaction
3. Application of Cyclodextrin in Asymmetric Reaction
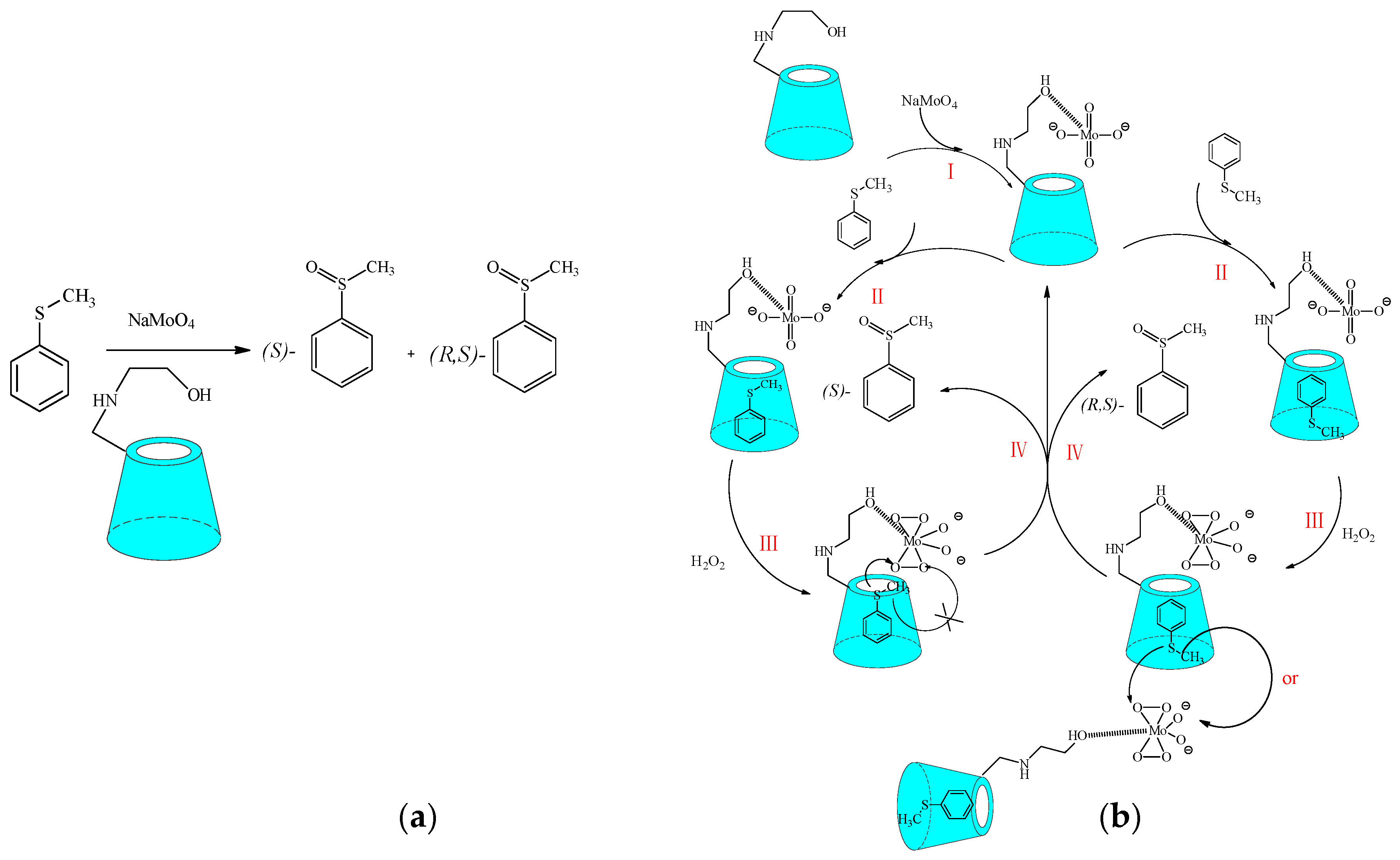
3.1. Asymmetric Oxidation Reaction
3.2. Asymmetric Reduction Reaction
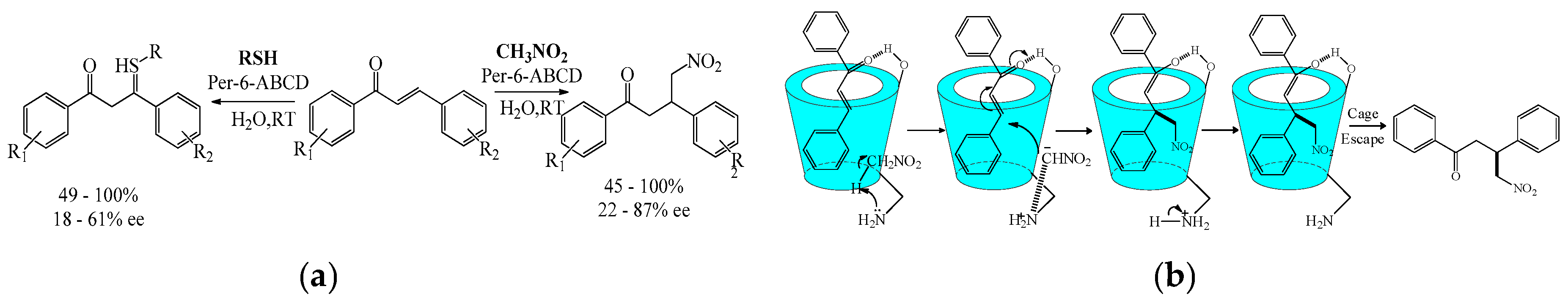
3.3. Asymmetric Addition Reaction
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lehn, J.M. Supramolecular chemistry. Science 1993, 260, 1762–1763. [Google Scholar] [CrossRef] [PubMed]
- André, J.M. The Nobel Prize in chemistry 2013. Chem. Int. 2014, 36, 2–7. [Google Scholar] [CrossRef]
- The 2016 Nobel Prize in Chemistry. Available online: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2016/press.html (accessed on 5 October 2016).
- Leigh, D.A. Genesis of the Nanomachines: The 2016 Nobel Prize in Chemistry. Angew. Chem. Int. Ed. 2016, 55, 14506–14508. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. The 2016 Nobel Prize for Chemistry, Awarded For: “The Design and Synthesis of Molecular Machines”. Sci. Prog. 2016, 99, 452–454. [Google Scholar] [CrossRef] [PubMed]
- De, P.E.; Cereda, C.M.; Fraceto, L.F.; de Araújo, D.R.; Franz-Montan, M.; Tofoli, G.R.; Ranali, J.; Volpato, M.C.; Groppo, F.C. Micro and nanosystems for delivering local anesthetics. Expert Opin. Drug Deliv. 2012, 9, 1505–1524. [Google Scholar]
- Ain, S.; Kumar, B.; Pathak, K. Cyclodextrin: Versatile carrier in drug formulations and delivery systems. Int. J. Pharm. Chem. Biol. S 2015, 5, 583–598. [Google Scholar]
- Cui, L.; Liu, Y.; Liu, T.; Yahong, Y.; Tianli, Y.; Rui, C.; Zhouli, W. Extraction of epigallocatechin gallate and epicatechin gallate from tea leaves using β-Cyclodextrin. J. Food Sci. 2017, 82, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Concheiro, A.; Alvarez-Lorenzo, C. Chemically cross-linked and grafted cyclodextrin hydrogels: From nanostructures to drug-eluting medical devices. Adv. Drug Deliv. Rev. 2013, 65, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Gan, C.; Shao, W.; Xing, Z.; Yong, C. Effects of cyclodextrins on the structure of LDL and its susceptibility to copper-induced oxidation. Eur. J. Pharm. Sci. 2016, 91, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Rathi, V.K.; Kesselheim, A.S.; Ross, J.S. The US Food and Drug Administration 515 Program Initiative: Addressing the evidence gap for widely used, high-risk cardiovascular devices? JAMA Cardiol. 2016, 1, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, Q.; Bi, Y.; Chen, X.; Liu, L.; Ruan, C.; Zhao, Z.; Jiang, C. Supramolecular amphiphiles based on cyclodextrin and hydrophobic drugs. J. Mater. Chem. B 2017, 5, 2644–2654. [Google Scholar] [CrossRef]
- Rutenberg, R.; Leitus, G.; Fallik, E.; Poverenov, E. Discovery of a non-classic host guest complexation mode in a β-cyclodextrin/propionic acid model. Chem. Commun. 2016, 52, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Sugiyama, T.; Ueno, A. New chemosensor for larger guests based on modified cyclodextrin bearing seven hydrophobic chains each with a hydrophilic end group. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 83–87. [Google Scholar] [CrossRef]
- Roy, M.C.; Roy, M.N. Investigation of Inclusion Complex formed by Ionic Liquid and β-Cyclodextrin through Hydrophilic and Hydrophobic Interactions. RSC Adv. 2015, 5, 56717–56723. [Google Scholar] [CrossRef]
- Cavalieri, F.; El, H.A.; Chiessi, E.; Paradossi, G.; Villa, R.; Zaffaroni, N. Tethering functional ligands onto shell of ultrasound active polymeric microbubbles. Biomacromolecules 2006, 7, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Hapiot, F.; Bricout, H.; Menuel, S.; Tilloy, S.; Monflier, E. Recent breakthroughs in aqueous cyclodextrin-assisted supramolecular catalysis. Catal. Sci. Technol. 2014, 4, 1899–1908. [Google Scholar] [CrossRef]
- Reetz, M.T.; Momotake, A.; Frömbgen, C. Chemoselective Reduction of Halo-Nitro Aromatic Compounds by β-Cyclodextrin-Modified Transition Metal Catalysts in a Biphasic System. Synthesis 1999, 9, 1555–1557. [Google Scholar] [CrossRef]
- Chen, H.Y.; Ji, H.B. β-cyclodextrin promoted oxidation of cinnamaldehyde to natural benzaldehyde in water. Chin. J. Chem. Eng. 2011, 19, 972–977. [Google Scholar] [CrossRef]
- Ji, H.B.; Shi, D.P.; Shao, M.; Li, Z.; Wang, L.F. Transition metal free and substrate-selective oxidation of alcohols using water as an only solvent in the presence of β-cyclodextrin. Tetrahedron Lett. 2005, 46, 2517–2520. [Google Scholar] [CrossRef]
- Lopez, O.L.; Marinescu, L.; Bols, M. New cup-shaped α-cyclodextrin derivatives and a study of their catalytic properties in oxidation reactions. Tetrahedron 2007, 63, 8872–8880. [Google Scholar] [CrossRef]
- Saghatforoush, L.; Hasanzadeh, M.; Shadjou, N. β-cyclodextrin/graphene oxide grafted sulfonic acid: Application for electro-oxidation and determination of cadaverine in fish samples. J. Electroanal. Chem. 2014, 714–715, 79–84. [Google Scholar] [CrossRef]
- Shi, D.P.; Ji, H.B. β-cyclodextrin promoted oxidation of aldehydes to carboxylic acids in water. Chin. Chem. Lett. 2009, 40, 139–142. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, H.; Zhou, X.; Ji, H. Enhanced catalytic activity and recyclability for oxidation of cinnamaldehyde catalysed by β-cyclodextrin cross-linked with chitosan. Supramol. Chem. 2013, 25, 233–245. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, H.; Zhou, X.; Ji, H. Mechanism into selective oxidation of cinnamaldehyde using β-cyclodextrin polymer as phase-transfer catalyst. Tetrahedron 2012, 68, 5912–5919. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Yao, X.; Fang, Y.; Chen, H.; Ji, H. β-cyclodextrin grafted on lignin as inverse phase transfer catalyst for the oxidation of benzyl alcohol in H2O. Tetrahedron 2016, 72, 1773–1781. [Google Scholar] [CrossRef]
- Chen, H.; Ji, H. Effect of substitution degree of 2-hydroxypropyl-β-cyclodextrin on the alkaline hydrolysis of cinnamaldehyde to benzaldehyde. Supramol. Chem. 2014, 26, 796–803. [Google Scholar] [CrossRef]
- PospíŠil, L.; Sokolová, R.; Hromadová, M.; Giannarelli, S.; Fuoco, R.; Colombini, M.P. Inclusion complex of fungicide vinclozoline and β-cyclodextrin: The influence of host–guest interaction on the reduction mechanism. J. Electroanal. Chem. 2001, 517, 28–36. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Zhao, F.; Lan, G.; Li, L.; Liu, Y.; Si, Y.; Jiang, Y.; Yang, B.; Yang, R. Palladium-modified functionalized cyclodextrin as an efficient and recyclable catalyst for reduction of nitroarenes. RSC Adv. 2016, 6, 7950–7954. [Google Scholar] [CrossRef]
- Kakroudi, M.A.; Kazemi, F.; Kaboudin, B. β-cyclodextrin–TiO2: Green nest for reduction of nitroaromatic compounds. RSC Adv. 2014, 4, 52762–52769. [Google Scholar] [CrossRef]
- Yun, L.U.; Liu, J.; Garry, D.; Liu, D.; Liu, B.O. Reduction of mononitroarenes by hydroxide ion in water catalyzed by β-cyclodextrin: Enhanced reactivity of hydroxide ion. Tetrahedron Lett. 2006, 47, 4597–4599. [Google Scholar]
- Krishnaveni, N.S.; Surendra, K.; Rao, K.R. Study of the Michael addition of β-cyclodextrin-thiol complexes to conjugated alkenes in water. Chem. Commun. 2005, 36, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Surendra, K.; Krishnaveni, N.S.; Reddy, M.A.; Nageswar, Y.V.D.; Rao, K.R. Mild oxidation of alcohols with o-iodoxybenzoic acid (IBX) in water/acetone mixture in the presence of β-cyclodextrin. J. Org. Chem. 2003, 68, 2058–2059. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, B.; Kumar, V.P.; Sridhar, R.; Reddy, V.P.; Rao, K.R. β-cyclodextrin-promoted addition of benzeneselenol to conjugated alCkenes in water. Helv. Chim. Acta 2009, 92, 1080–1084. [Google Scholar] [CrossRef]
- Surendra, K.; Krishnaveni, N.S.; Sridhar, R.; Rao, K.R. β-cyclodextrin promoted Aza-Michael addition of amines to conjugated alkenes in water. Tetrahedron Lett. 2006, 47, 2125–2127. [Google Scholar] [CrossRef]
- Vassilov, A.; Zlatkova, M. Cyclodextrins in polymer modification: Diels-Alder addition of cyclopentadiene/methylated-β-cyclodextrin complex on unsaturated polyester and formation of a new type of polypseudorotaxane. Macromol. Rapid Commun. 2005, 26, 40–45. [Google Scholar]
- Tao, F.; Wang, Q. Aqueous radical addition-coupling polymerization using nitroso benzene/cyclodextrin complex for synthesis of hydrophilic periodic polymer. RSC Adv. 2015, 5, 46007–46010. [Google Scholar] [CrossRef]
- Girish, Y.R.; Kumar, K.S.S.; Thimmaiah, K.N.; Rangappa, K.S.; Shashikanth, S. ZrO2-β-cyclodextrin catalyzed synthesis of 2,4,5-trisubstituted imidazoles and 1,2-disubstituted benzimidazoles under solvent free conditions and evaluation of their antibacterial study. RSC Adv. 2015, 5, 75533–75546. [Google Scholar] [CrossRef]
- Ge, T.; Zou, C.; Zuo, C. Monitoring the effects of hydroxypropyl-β-cyclodextrin as a biomimic catalyst (phase transfer catalyst) for glycidyl monostearate synthesis. Ind. Eng. Chem. Res. 2015, 54, 1723–1730. [Google Scholar] [CrossRef]
- Galia, A.; Scialdone, O.; Spanò, T.; Grazia Valenti, M.; Grignard, B.; Lecomte, P.; Monflier, E.; Tilloy, S.; Rousseau, C. Ring opening polymerization of ε-caprolactone in the presence of wet β-cyclodextrin: Effect of the operative pressure and of water molecules in the β-cyclodextrin cavity. RSC Adv. 2016, 6, 90290–90299. [Google Scholar] [CrossRef]
- Kiasat, A.R.; Nazari, S. Magnetic nanoparticles grafted with β-cyclodextrin–polyurethane polymer as a novel nanomagnetic polymer brush catalyst for nucleophilic substitution reactions of benzyl halides in water. J. Mol. Catal. A Chem. 2012, 365, 80–86. [Google Scholar] [CrossRef]
- Kiasat, A.R.; Zarinderakht, N.; Sayyahi, S. β-Cyclodextrin immobilized onto dowex resin: A unique microvessel and heterogeneous catalyst in nucleophilic substitution reactions. Chin. J. Chem. 2012, 30, 699–702. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Chen, L.Q.; Tao, J.; Shen, J.L.; Gong, D.Y.; Yun, R.R.; Cheng, Y. Effective cleavage of phosphodiester promoted by the zinc(II) and copper(II) inclusion complexes of β-cyclodextrin. J. Inorg. Biochem. 2016, 163, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Andrés, G.O.; Rossi, R.H.D.; To, D.; Rúveda, E.; Rossi, R.A. Mechanism of phthalate ester hydrolysis in water and in cyclodextrin mediated reactions. Arkivoc 2003, 36, 127–138. [Google Scholar]
- Drabowicz, J.; Mikołajczyk, M. Asymmetric Oxidation of Sulfides to Sulfoxides Catalyzed by β-Cyclodextrin. Phosphorous Sulfur Relat. Elem. 1984, 21, 245–248. [Google Scholar] [CrossRef]
- Anthony, W. Czarnik Cyclodextrin-mediated chiral sulfoxidations. J. Org. Chem. 1984, 49, 924–927. [Google Scholar]
- Shen, H.M.; Ji, H.B. Amino alcohol-modified β-cyclodextrin inducing biomimetic asymmetric oxidation of thioanisole in water. Carbohydr. Res. 2012, 354, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Mojr, V.; Ínsky, M.B.; Cibulka, R.; Kraus, T. Alloxazine- cyclodextrin conjugates for organocatalytic enantioselective sulfoxidations. Org. Biomol. Chem. 2011, 9, 7318–7326. [Google Scholar] [CrossRef] [PubMed]
- Mojr, V.; Herzig, V.; Ínsky, M.B.; Cibulka, R.; Kraus, T. Flavin-cyclodextrin conjugates as catalysts of enantioselective sulfoxidations with hydrogen peroxide in aqueous media. Chem. Commun. 2010, 46, 7599–7601. [Google Scholar] [CrossRef] [PubMed]
- Park, K.K.; Sim, W.J.; Park, J.W. Asymmetric induction by β-cyclodextrins in NaBH4 reduction of ketones. J. Incl. Phenom. Macrocycl. Chem. 1997, 27, 41–48. [Google Scholar] [CrossRef]
- Deratani, A.; Renard, E. Substituent effects in the enantioselective reduction of acetophenones with NaBH4 in the presence of β-cyclodextrin. Chirality 1994, 6, 658–664. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, X.; Liu, N. Interaction of β-cyclodextrin as catalyst with acetophenone in asymmetric reaction: A theoretical survey. J. Mol. Model. 2014, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakajima, M.; Maeda, Y.; Uemura, S.; Takekuma, S.I.; Takekuma, H.; Yoshida, Z.I. γ-cyclodextrin-bicapped C60 -mediated asymmetric reduction of ketones with NaBH4. Bull. Chem. Soc. Jpn. 2004, 77, 2047–2050. [Google Scholar] [CrossRef]
- Jouffroy, M.; Sémeril, D.; Armspach, D.; Matt, D. Phosphane-Phosphite Chelators Built on a α-Cyclodextrin Scaffold: Application in Rh-Catalysed Asymmetric Hydrogenation and Hydroformylation. Eur. J. Org. Chem. 2013, 2013, 6069–6077. [Google Scholar] [CrossRef]
- Suresh, P.; Pitchumani, K. Per-6-amino-β-cyclodextrin catalyzed asymmetric Michael addition of nitromethane and thiols to chalcones in water. Tetrahedron Asymmetr. 2008, 19, 2037–2044. [Google Scholar] [CrossRef]
- Kanagaraj, K.; Pitchumani, K. Per-6-amino-β-cyclodextrin as a Chiral Base Catalyst Promoting One-Pot Asymmetric Synthesis of 2-Aryl-2,3-dihydro-4-quinolones. J. Org. Chem. 2013, 78, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Shen, H.; Yang, Z.; Ji, H. Biomimetic asymmetric Michael addition reactions in water catalyzed by amino-containing β-cyclodextrin derivatives. Chin. J. Catal. 2016, 37, 1227–1234. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, G. ChemInform Abstract: Direct Asymmetric Aldol reactions in aqueous media catalyzed by a β-cyclodextrin-proline conjugate with a urea linker. Tetrahedron Lett. 2015, 56, 243–246. [Google Scholar] [CrossRef]
- Doyagüez, E.G.; Fernández-Mayoralas, A. Proline–cyclodextrin conjugates: Synthesis and evaluation as catalysts for aldol reaction in water. Tetrahedron 2012, 68, 7345–7354. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, J.; Liu, Y.; Jiao, C.; Li, M.; Zhang, Y. β-cyclodextrin-immobilized (4S)-phenoxy-(S)-proline as a catalyst for direct asymmetric aldol reactions. Chirality 2005, 17, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Pan, J.H.; Wang, S.Y.; Fang, X.U.; Tao, Y.W.; She, Z.G.; Lin, Y.C. Asymmetric synthesis of 2-methoxy-2-methylchroman-7-ol in solid-state β-cyclodextrin complexes. Chin. J. Chem. 2008, 26, 741–744. [Google Scholar]





















© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, C.C.; Tian, B.R.; Zhao, T.; Huang, Q.; Wang, Z.Z. Cyclodextrin-Catalyzed Organic Synthesis: Reactions, Mechanisms, and Applications. Molecules 2017, 22, 1475. https://doi.org/10.3390/molecules22091475
Bai CC, Tian BR, Zhao T, Huang Q, Wang ZZ. Cyclodextrin-Catalyzed Organic Synthesis: Reactions, Mechanisms, and Applications. Molecules. 2017; 22(9):1475. https://doi.org/10.3390/molecules22091475
Chicago/Turabian StyleBai, Chang Cai, Bing Ren Tian, Tian Zhao, Qing Huang, and Zhi Zhong Wang. 2017. "Cyclodextrin-Catalyzed Organic Synthesis: Reactions, Mechanisms, and Applications" Molecules 22, no. 9: 1475. https://doi.org/10.3390/molecules22091475




