Early Embryogenesis of Brown Alga Fucus vesiculosus L. is Characterized by Significant Changes in Carbon and Energy Metabolism
Abstract
:1. Introduction
2. Results
2.1. General Description of Metabolic Profiles
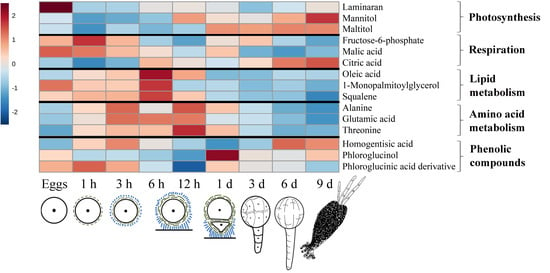
2.2. Changes in Metabolite Profiles during Fertilization and Zygote Development
2.3. Changes in Metabolite Profiles in 1–9 Days Old Embryos
3. Discussion
3.1. General Considerations
3.2. Zygote Development
3.3. Embryogenesis after the First Zygote Division
4. Materials and Methods
4.1. Plant Material Collection and Culturing
4.2. Sample Preparation
4.3. Metabolite Profiling
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, K.; McKinley, K.R. Use of macroalgae for marine biomass production and CO2 remediation: A review. J. Appl. Phycol. 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Truus, K.; Vaher, M.; Taure, I. Algal biomass from Fucus vesiculosus (Phaeophyta): Investigation of the mineral and alginate components. Proc. Estonian Acad. Sci. Chem. 2001, 50, 95–103. [Google Scholar]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Characterization of the biosorption of cadmium, lead and copper with the brown alga Fucus vesiculosus. J. Hazard. Mater. 2008, 158, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H. Brown marine algae: A survey of therapeutic potentials. Altern. Complement. Ther. 2003, 9, 29–33. [Google Scholar] [CrossRef]
- McLachlan, J.; Bidwell, R.G.S. Photosynthesis of eggs, sperm, zygotes, and embryos of Fucus serratus. Can. J. Bot. 1978, 56, 371–373. [Google Scholar] [CrossRef]
- Bisgrove, S.R.; Kropf, D.L. Cell wall deposition during morphogenesis in fucoid algae. Planta 2001, 212, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Polevoi, V.V.; Tarakhovskaya, E.R.; Maslov, Y.I.; Polevoi, A.V. Role of auxin in induction of polarity in Fucus vesiculosus zygote. Russ. J. Develop. Biol. 2003, 34, 360–364. [Google Scholar] [CrossRef]
- Homblé, F.; Léonetti, M. Emergence of symmetry breaking in fucoid zygotes. Trends Plant Sci. 2007, 12, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Tarakhovskaya, E.R. Mechanisms of bioadhesion of macrophytic algae. Russ. J. Plant Physiol. 2014, 61, 23–30. [Google Scholar] [CrossRef]
- Quatrano, R.S. Development of cell polarity. Ann. Rev. Plant. Physiol. 1978, 29, 487–510. [Google Scholar] [CrossRef]
- Kropf, D.L.; Bisgrove, S.R.; Hable, W.E. Establishing a growth axis in fucoid algae. Trends Plant Sci. 1999, 4, 490–494. [Google Scholar] [CrossRef]
- Quatrano, R.S. Developmental biology: Development in marine organisms. In Experimental Marine Biology; Mariscal, R.N., Ed.; Academic Press: New York, NY, USA; London, UK, 1974; pp. 303–346. [Google Scholar]
- Kropf, D.L. Induction of polarity in fucoid zygotes. Plant Cell 1997, 9, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Bisgrove, S.R.; Henderson, D.C.; Kropf, D.L. Asymmetric division in fucoid zygotes is positioned by telophase nuclei. Plant Cell 2003, 15, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Bouget, F.Y.; Berger, F.; Brownlee, C. Position dependent control of cell fate in the Fucus embryo: Role of intercellular communication. Development 1998, 125, 1999–2008. [Google Scholar] [PubMed]
- Nienburg, W. Die Entwicklung der Keimlinge von Fucus vesiculosus und ihre Bedeutung für die Phylogenie der Phaeophyceen. Wiss. Meer. Ab. Kiel. 1931, 1, 52–62. [Google Scholar]
- Galun, E.; Torrey, J.G. Initiation and suppression of apical hairs of Fucus embryos. Develop. Biol. 1969, 19, 447–459. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Kang, E.J.; Kim, K.Y.; Garbary, D.J. Influence of phytohormones on morphology and chlorophyll a fluorescence parameters in embryos of Fucus vesiculosus L. (Phaeophyceae). Russ. J. Plant Physiol. 2013, 60, 176–183. [Google Scholar] [CrossRef]
- Vadas, R.L., Sr.; Johnson, S.; Norton, T.A. Recruitment and mortality of early post-settlement stages of benthic algae. Br. Phycol. J. 1992, 27, 331–351. [Google Scholar] [CrossRef]
- Van Alstyn, K.L.; Whitman, S.L.; Ehlig, J.M. Differences in herbivore preferences, phlorotannin production, and nutritional quality between juvenile and adult tissues from marine brown algae. Mar. Biol. 2001, 139, 201–210. [Google Scholar] [CrossRef]
- Allen, D.A.; Jacobsen, L.; Joaquin, J.; Jaffe, L.F. Ionic concentrations in developing Pelvetia eggs. Develop. Biol. 1972, 27, 538–545. [Google Scholar] [CrossRef]
- Robinson, K.R.; Jaffe, L.F. Ion movements in a developing fucoid egg. Develop. Biol. 1973, 35, 349–361. [Google Scholar] [CrossRef]
- Michel, G.; Tonon, T.; Scornet, D.; Cock, J.M.; Kloareg, B. Central and storage carbon metabolism of the brown alga Ectocarpus siliculosus: Insights into the origin and evolution of storage carbohydrates in Eukaryotes. New Phytol. 2010, 188, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Gravot, A.; Dittami, S.M.; Rousvoal, S.; Lugan, R.; Eggert, A.; Collén, J.; Boyen, C.; Bouchereau, A.; Tonon, T. Diurnal oscillations of metabolite abundances and gene analysis provide new insights into central metabolic processes of the brown alga Ectocarpus siliculosus. New Phytol. 2010, 188, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Dittami, S.M.; Gravot, A.; Renault, D.; Goulitquer, S.; Eggert, A.; Bouchereau, A.; Boyen, C.; Tonon, T. Integrative analysis of metabolite and transcript abundance during the short-term response to saline and oxidative stress in the brown alga Ectocarpus siliculosus. Plant Cell Environ. 2011, 34, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Cock, J.M.; Sterck, L.; Rouze, P.; Scornet, D.; Allen, A.E.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.M.; Badger, J.H.; et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Holligan, P.M.; Drew, E.A. Routine analysis by gas-liquid chromatography of soluble carbohydrates in extracts of plant tissues. II. Quantitative analysis of standard carbohydrates, and the separation and estimation of soluble sugars and polyols from a variety of plant tissues. New Phytol. 1971, 70, 271–297. [Google Scholar] [CrossRef]
- Jones, A.L.; Harwood, J.L. Lipid metabolism in the brown marine algae Fucus vesiculosus and Ascophyllum nodosum. J. Exp. Bot. 1993, 44, 1203–1210. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Titlyanova, T.V. Fatty acids of marine algae from the pacific coast of North California. Bot. Mar. 2002, 45, 17–22. [Google Scholar] [CrossRef]
- Krivoruchko, Y.V.; Kanaan, H.M.; Kovalev, V.N. Chromato-mass-spectrometry analysis of the composition of Padina pavonica (L.) Gaill. and Fucus vesiculosus L. Pharmacom 2010, 3, 32–35. (In Russian) [Google Scholar]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chemistry 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Gosch, B.J.; Paul, N.A.; de Nys, R.; Magnusson, M. Seasonal and within-plant variation in fatty acid content and composition in the brown seaweed Spatoglossum macrodontum (Dictyotales, Phaeophyceae). J. Appl. Phycol. 2014, 27, 387–398. [Google Scholar] [CrossRef]
- Schmid, M.; Stengel, D.B. Intra-thallus differentiation of fatty acid and pigment profiles in some temperate Fucales and Laminariales. J. Phycol. 2015, 51, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Ikawa, T.; Nisizawa, K. Incorporation of radioactive carbon from H14CO3− into sugar constituents by a brown alga, Eisenia bicyclis, during photosynthesis and its fate in the dark. Plant Cell Physiol. 1966, 7, 217–229. [Google Scholar] [CrossRef]
- Kamenarska, Z.; Gasic, M.J.; Zlatovic, M.; Rasovic, A.; Sladic, D.; Kljajic, Z.; Stefanov, K.; Seizova, K.; Najdenski, H.; Kujumgiev, A.; et al. Chemical composition of the brown alga Padina pavonia (L.) Gaill. from the Adriatic Sea. Bot. Mar. 2002, 45, 339–345. [Google Scholar] [CrossRef]
- Rickert, E.; Wahl, M.; Link, H.; Richter, H.; Pohnert, G. Seasonal variations in surface metabolite composition of Fucus vesiculosus and Fucus serratus from the Baltic Sea. PLoS ONE 2016, 11, e0168196. [Google Scholar] [CrossRef] [PubMed]
- Rosell, K.; Srivastava, L. Seasonal variations in total nitrogen, carbon and amino acids in Macrocystis integrifolia and Nereocystis luetkeana (Phaeophyta). J. Phycol. 1985, 21, 304–309. [Google Scholar] [CrossRef]
- Craigie, J.S. Storage products. In Algal Physiology and Biochemistry; Stewart, W.D.P., Ed.; University of California Press: Berkeley and Los Angeles, CA, USA, 1974; pp. 206–235. [Google Scholar]
- Graiff, A.; Ruth, W.; Udo Kragl, U.; Karsten, U. Chemical characterization and quantification of the brown algal storage compound laminarin—A new methodological approach. J. Appl. Phycol. 2016, 28, 533–543. [Google Scholar] [CrossRef]
- Kim, K.Y.; Jeong, H.J.; Main, H.P.; Garbary, D.J. Fluorescence and photosynthetic competency in single eggs and embryos of Ascophyllum nodosum (Phaeophyceae). Phycologia 2006, 45, 331–336. [Google Scholar] [CrossRef]
- Quatrano, R.S.; Stevens, P.T. Cell wall assembly in Fucus zygotes: 1. Characterization of the polysaccharide components. Plant Physiol. 1976, 58, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Major, K.M.; Davison, I.R. Influence of temperature and light on growth and photosynthetic physiology of Fucus evanescens (Phaeophyta) embryos. Eur. J. Phycol. 1998, 33, 129–138. [Google Scholar] [CrossRef]
- Reed, R.H.; Davison, I.R.; Chudek, J.A.; Foster, R. The osmotic role of mannitol in the Phaeophyta: An appraisal. Phycologia 1985, 24, 35–47. [Google Scholar] [CrossRef]
- Levring, T. Remarks on the submicroscopical structure of eggs and spermatozoids of Fucus and related genera. Physiol. Plant. 1952, 5, 528–539. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I. Description of the photosynthetic apparatus of Fucus vesiculosus L. in early embryogenesis. Biol. Bull. 2005, 32, 456–460. [Google Scholar] [CrossRef]
- Bidwell, R.G.S.; Ghosh, R. Photosynthesis and metabolism of marine algae V. Respiration and metabolism of C14-labelled glucose and organic acids supplied to Fucus vesiculosus. Can. J. Bot. 1963, 41, 155–163. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from algae. Biopolym. Online 2005, 6. [Google Scholar] [CrossRef]
- Michel, G.; Tonon, T.; Scornet, D.; Cock, J.M.; Kloareg, B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 2010, 188, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Tsekos, I. The sites of cellulose synthesis in algae: Diversity and evolution of cellulose-synthesizing enzyme complexes. J. Phycol. 1999, 35, 635–655. [Google Scholar] [CrossRef]
- Loewus, F.A.; Murthy, P.P.N. Myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Schoenwaelder, M.E.A.; Clayton, M.N. Secretion of phenolic substances into the zygote wall and cell plate in embryos of Hormosira and Acrocarpia (Fucales, Phaeophyceae). J. Phycol. 1998, 34, 969–980. [Google Scholar] [CrossRef]
- Hable, W.E.; Kropf, D.L. Roles of secretion and cytoskeleton in cell adhesion and polarity establishment in Pelvetia compressa zygotes. Develop. Biol. 1998, 198, 45–65. [Google Scholar] [CrossRef]
- Steer, M.W.; Steer, J.M. Pollen tube tip growth. New Phytol. 1989, 111, 323–358. [Google Scholar] [CrossRef]
- Ragan, M.A.; Glombitza, K.W. Phlorotannins, brown algal polyphenols. In Progress in Phycological Research; Round, F.E., Chapman, D.J., Eds.; Biopress Ltd.: Amsterdam, The Netherlands, 1986; Volume 4, pp. 130–241. [Google Scholar]
- Parys, S.; Kehraus, S.; Krick, A.; Glombitza, K.W.; Carmeli, S.; Klimo, K.; Gerhäuser, C.; König, G.M. In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 2010, 71, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Potin, P.; Leblanc, C. Phenolic-based adhesives of marine brown algae. In Biological Adhesives; Smith, A.M., Callow, J.A., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; pp. 105–124. [Google Scholar]
- Schoenwaelder, M.E.A.; Wiencke, C. Phenolic compounds in the embryo development of several northern hemisphere fucoids. Plant Biol. 2000, 2, 24–33. [Google Scholar] [CrossRef]
- Meslet-Cladiere, L.; Delage, L.; Leroux, C.J.; Goulitquer, S.; Leblanc, C.; Creis, E.; Gall, E.A.; Stiger-Pouvreau, V.; Czjzek, M.; Potin, P. Structure/function analysis of a type iii polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell 2013, 25, 3089–3103. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, L.F.; Neuscheler, W. On the mutual polarization of nearby pairs of Fucaceous eggs. Develop. Biol. 1969, 19, 549–565. [Google Scholar] [CrossRef]
- Hutschenreuther, A.; Kiontke, A.; Birkenmeier, G.; Birkemeyer, C. Comparison of extraction conditions and normalization approaches for cellular metabolomics of adherent growing cells with GCMS. Anal. Methods 2012, 4, 1959–1963. [Google Scholar] [CrossRef]
- Kovàts, E. Characterization of organic compounds by gas chromatography. Part 1. Retention indices of aliphatic halides, alcohols, aldehydes and ketones. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Stein, S.E. An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J. Am. Soc. Mass. Spectrom. 1999, 10, 770–781. [Google Scholar] [CrossRef]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmüller, E.; Dörmann, P.; Weckwerth, W.; Gibon, Y.; Willmitzer, M.S.L.; et al. Steinhauser D. GMD@CSB.DB: The Golm Metabolome Database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Wiencke, C. Seasonal changes in C, N and major organic compounds and their significance to morpho-functional processes in the endemic Antarctic brown alga Ascoseira mirabilis. Polar Biol. 1998, 19, 115–124. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinformat. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| PC 1 | PC 2 | PC 3 |  |
| Squalene (−) | Trisaccharide RI 3415 (−) | Phloroglucinic acid derivative (+) | |
| Sugar RI 3527 (+) | Serine (−) | Glucose-6-phosphate (+) | |
| Polyol RI 3514 (+) | Disaccharide RI 2669 (−) | 1-Octadecanol (+) | |
| Maltitol (+) | Glucose (−) | Glycine (−) | |
| Disaccharide RI 2789 (+) | Myo-Inositol (+) | Dodecanoic acid methylester (+) | |
| Fumaric acid (−) | Fructose (+) | Myo-Inositol-1-phosphate (+) | |
| Galactose (+) | Citric acid (+) | Eicosanoic acid (+) | |
| Xylose (−) | Trisaccharide RI 3447 (−) | Threonine (−) | |
| Arabinose (+) | Aspartic acid (−) | Fructose-6-phosphate (+) | |
| Oleic acid (−) | 4-Hydroxybenzoic acid (+) | Citric acid (−) | |
| 1-Monopalmitoylglycerol (−) | Threitol (−) | Malic acid (+) | |
| Disaccharide RI 2851 (+) | Alanine (−) | Isoleucine (−) | |
| Phenylalanine (+) | Pentitol RI 1685 (+) | β-alanine (−) | |
| Linoleic acid (−) | Glyceric acid (+) | 1-Eicosanol (+) | |
| Polyol RI 3482 (+) | Pipecolic acid (−) | Oleic acid (−) | |
| Arachidonic acid (−) | Homogentisic acid (+) | Linoleic acid (−) | |
| Sucrose (−) | Maltose (+) | Glutamic acid (−) | |
| Succinic acid (+) | Phytol (+) | Arachidonic acid (−) | |
| Phosphoric acid (+) | Polyol RI 3466 (−) | Pipecolic acid (−) | |
| Polyol RI 3458 (+) | Tocopherol γ (+) | Myristic acid (−) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarakhovskaya, E.; Lemesheva, V.; Bilova, T.; Birkemeyer, C. Early Embryogenesis of Brown Alga Fucus vesiculosus L. is Characterized by Significant Changes in Carbon and Energy Metabolism. Molecules 2017, 22, 1509. https://doi.org/10.3390/molecules22091509
Tarakhovskaya E, Lemesheva V, Bilova T, Birkemeyer C. Early Embryogenesis of Brown Alga Fucus vesiculosus L. is Characterized by Significant Changes in Carbon and Energy Metabolism. Molecules. 2017; 22(9):1509. https://doi.org/10.3390/molecules22091509
Chicago/Turabian StyleTarakhovskaya, Elena, Valeriya Lemesheva, Tatiana Bilova, and Claudia Birkemeyer. 2017. "Early Embryogenesis of Brown Alga Fucus vesiculosus L. is Characterized by Significant Changes in Carbon and Energy Metabolism" Molecules 22, no. 9: 1509. https://doi.org/10.3390/molecules22091509
APA StyleTarakhovskaya, E., Lemesheva, V., Bilova, T., & Birkemeyer, C. (2017). Early Embryogenesis of Brown Alga Fucus vesiculosus L. is Characterized by Significant Changes in Carbon and Energy Metabolism. Molecules, 22(9), 1509. https://doi.org/10.3390/molecules22091509