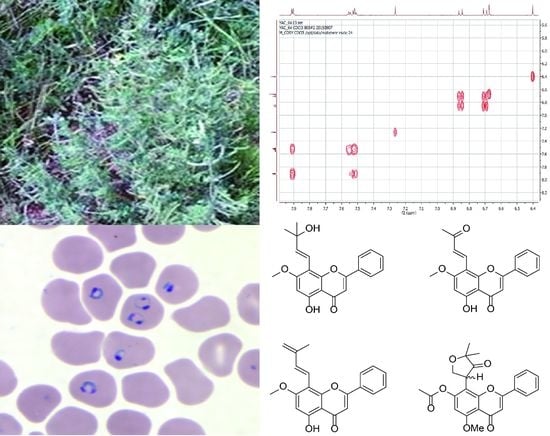
Four Prenylflavone Derivatives with Antiplasmodial Activities from the Stem of Tephrosia purpurea subsp. leptostachya
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Extraction and Isolation
3.4. In Vitro Antiplasmodial Activity
3.5. Cell Culture
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Queiroz, R.T.; Goulart de Azevedo Tozzi, A.M.; Lewis, G.P. Seed morphology: An addition to the taxonomy of Tephrosia (Leguminosae, Papilionoideae, Millettieae) from South America. Plant Syst. Evol. 2013, 299, 459–470. [Google Scholar] [CrossRef]
- Al-Ghamdi, F.A.; Al-Zahrani, R.M. Seed morphology of some species of Tephrosia Pers. (Fabaceae) from Saudi Arabia Identification of species and systematic significance. Feddes Repert. 2010, 121, 59–65. [Google Scholar] [CrossRef]
- Hosni, H.; El-Karemy, Z. Systematic revision of Leguminosae in Egypt. 1. Tephrosia Pers. Sendtnera 1993, 1, 245–257. [Google Scholar]
- Bosman, M.T.M.; De Haas, A.J.P. A revision of the genus Tephrosia (Leguminosae-Papilionoideae) in Malesia. Blumea-Biodivers. Evol. Biogeogr. Plants 1983, 28, 421–487. [Google Scholar]
- Gillett, J.B. Notes on Tephrosia in Tropical Africa. Kew Bull. 1958, 13, 111. [Google Scholar] [CrossRef]
- Neuwinger, H.D. African Traditional Medicine; MedPharm Scientific Publishers: Stuttgart, Germany, 2000; pp. 515–516. [Google Scholar]
- Kokwaro, J.O. Medicinal Plants of East Africa, 3rd ed.; University of Nairobi Press: Nairobi, Kenya, 2009; pp. 185–187. [Google Scholar]
- Chang, L.C.; Daniel, C.; Song, L.L.; Fransworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D. Absolute configuration of novel bioactive flavonoids from Tephrosia purpurea. Org. Lett. 2000, 2, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.; Abd el-Razek, M.H.; Nagashima, F.; Asakawa, Y.; Pare, P.W. Rare prenylated flavonoids from Tephrosia purpurea. Phytochemistry 2009, 70, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.U.; Ali, Z.; Hussaini, S.R.; Iqbal, F.; Zahid, M.; Abbas, M.; Saba, N. Flavonoids of Tephrosia purpurea. Fitoterapia 1999, 70, 443–445. [Google Scholar] [CrossRef]
- Sinha, B.; Natu, A.A.; Nanavati, D.D. Prenylated favonoids from Tephrosia purpurea seeds. Phytochemistry 1982, 21, 1468–1470. [Google Scholar] [CrossRef]
- Juma, W.P.; Akala, H.M.; Eyase, F.L.; Muiva, L.M.; Heydenreich, M.; Okalebo, F.A.; Gitu, P.M.; Peter, M.G.; Walsh, D.S.; Imbuga, M.; et al. Terpurinflavone: An antiplasmodial flavone from the stem of Tephrosia purpurea. Phytochem. Lett. 2011, 4, 176–178. [Google Scholar] [CrossRef]
- Muiva-Mutisya, L.; Bernard, M.; Matthias, H.; Andreas, K.; Akala, H.M.; Derese, S.; Omosa, L.K.; Yusuf, A.O.; Edwin, K.; Yenesew, A. 6α-Hydroxy-α-toxicarol and (+)-tephrodin with antiplasmodial activities from Tephrosia species. Phytochem. Lett. 2014, 10, 179–183. [Google Scholar] [CrossRef]
- Gulecha, V.; Sivakuma, T. Anticancer activity of Tephrosia purpurea and Ficus religiosa using MCF 7 cell lines. Asian Pac. J. Trop. Med. 2011, 4, 526–529. [Google Scholar] [CrossRef]
- Sandhya, S.; Venkata, K.R.; Vinod, K.R.; Rsnakk, C. Assessment of in vitro antacid activity of different root extracts of Tephrosia purpurea (L) Pers by modified artificial stomach model. Asian Pac. J. Trop. Med. 2012, 2, S1487–S1492. [Google Scholar] [CrossRef]
- Jain, A.; Nahata, A.; Santram, L.; Singhai, A.K. Effects of Tephrosia purpurea and Momordica dioica on streptozotocin-induced diabetic nephropathy in rats. Biomed. Prev. Nutr. 2014, 4, 383–389. [Google Scholar] [CrossRef]
- Shenoy, S.; Shwetha, K.; Prabhu, K.; Maradi, R.; Bairy, K.L.; Shanbhag, T. Evaluation of antiinflammatory activity of Tephrosia purpurea in rats. Asian Pac. J. Trop. Med. 2010, 3, 193–195. [Google Scholar] [CrossRef]
- Khatri, A.; Garg, A.; Agrawal, S.S. Evaluation of hepatoprotective activity of aerial parts of Tephrosia purpurea L. and stem bark of Tecomella undulata. J. Ethnopharm. 2009, 122, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chinniah, A.; Mohapatra, S.; Goswami, S.; Mahapatra, A.; Kar, S.K.; Mallavadhani, U.V.; Das, P.K. On the potential of Tephrosia purpurea as anti-Helicobacter pylori agent. J. Ethnopharm. 2009, 124, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.A.; Waterman, P.G. 8-C-Prenylflavonoids from the seed of Tephrosia bracteolata. Phytochemistry 1981, 20, 1719–1720. [Google Scholar] [CrossRef]
- Jang, D.S.; Park, E.J.; Kang, Y.-H.; Hawthorne, M.E.; Vigo, J.S.; Graham, J.G.; Cabieses, F.; Fong, H.H.; Mehta, R.G.; Pezzuto, J.M. Potential cancer chemopreventive flavonoids from the stems of Tephrosia toxicaria. J. Nat. Prod. 2003, 66, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Bedane, K.G.; Kusari, S.; Masesane, I.B.; Spiteller, M.; Majinda, R.R. Flavanones of Erythrina livingstoniana with antioxidant properties. Fitoterapia 2016, 108, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Waterman, P.G.; Khalid, S.A. The major flavonoids of the seed of Tephrosia apollinea. Phytochemsitry 1980, 19, 909–915. [Google Scholar] [CrossRef]
- Lin, C.-F.; Liu, Y.-W.; Kuo, Y.-H.; Shen, C.-C.; Chiou, W.-F.; Chen, C.-C. Two new isoflavones from the tubers of Apios taiwanianus. Phytochem. Lett. 2016, 15, 164–167. [Google Scholar] [CrossRef]
- Yuldashev, M.P.; Batirov, E.S.; Vdovin, A.D.; Abdullaev, N.D. Flavonoids from the aerial parts of Glycyrrhiza glabra L. Izv. Minist. Obraz. Nauki Resp. Kaz., Nats. Akad. Nauk Resp. Kaz., Ser. Khim. 2000, 2, 67–71. [Google Scholar]
- Chen, Y.-L.; Wang, Y.-S.; Lin, Y.-L.; Munakata, K.; Ohta, K. Obovatin, obovatin methyl ether and obovatachalcone, new piscicidal flavonoids from Tephrosia obovata. Agric. Biol. Chem. 1978, 42, 2431–2432. [Google Scholar] [CrossRef]
- Gao, J.Y.; Jiang, Y.L.; Niu, L.L.; Li, H.D.; Yin, W.P. Novel isoflavone from the cockroach Periplaneta americana. Chem. Nat. Compd. 2016, 52, 413–416. [Google Scholar] [CrossRef]
- Smalberger, T.; Vleggaar, R.; De Waal, H.L. Tachrosin: A new flavone from Tephrosia polystachyoides. J. S. Afr. Chem. Inst. 1971, 24, 1–12. [Google Scholar]
- Yin, R.; Han, K.; Heller, W.; Albert, A.; Dobrev, P.I.; Zazimalova, E.; Schaeffner, A.R. Kaempferol 3-O-rhamnoside-7-O-rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in Arabidopsis shoots. New Phytol. 2014, 201, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Raya-Gonzalez, D.; Pamatz-Bolanõs, T.; Rio-Torres, R.E.D.; Martinez-Munõz, R.E.; Ron-Echeverria, O.; Martinez-Pacheco, M.M. D-(+)-Pinitol, a component of the heartwood of Enterolobium cyclocarpum (Jacq.) Griseb. Z. Naturforsch. C 2008, 63, 922. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.R.; Lopes, L.M.X. Antiplasmodial natural products. Molecules 2011, 16, 2146–2190. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Okoth, D.A.; Akala, H.M.; Johnson, J.D.; Koorbanally, N.A. Alkyl phenols, alkenyl cyclohexenones and other phytochemical constituents from Lannea rivae (chiov) Sacleux (Anacardiaceae) and their bioactivity. Med. Chem. Res. 2016, 25, 690–703. [Google Scholar] [CrossRef]
- Wong, V.K.; Li, T.; Law, B.Y.; Ma, E.D.; Yip, N.C.; Michelangeli, F.; Law, C.K.; Zhang, M.M.; Lam, K.Y.; Chan, P.L.; et al. Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 2013, 4, e720. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compounds 1, 4–11 are available from the authors. |

| Position | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δC (ppm) | δH, m (J in Hz) | HMBC (H→C) | δC | δH, m (J in Hz) | HMBC (H→C) | δC | δH, m (J in Hz) | HMBC (H→C) | |
| 2 | 164.2 | 164.6 | 164.2 | ||||||
| 3 | 105.5 | 6.57 s | C-2, C-4, C-4a, C-1′ | 106.2 | 6.74 s | C-2, C-4, C-4a, C-1′ | 105.5 | 6.71 s | C-2, C-4, C-4a, C-1′ |
| 4 | 182.9 | 182.6 | 183.0 | ||||||
| 4a | 105.2 | 105.4 | 105.3 | ||||||
| 5 | 161.3 | 164.2 | 161.4 | ||||||
| 5-OH | 13.08 s | C-4a, C-5, C-6 | 13.41 s | C-4a, C-5, C-6 | 13.11 s | C-4a, C-5, C-6 | |||
| 6 | 95.3 | 6.40 s | C-4a, C-5, C-7, C-8 | 95.6 | 6.40 s | C-4a, C-5, C-7, C-8 | 95.4 | 6.45 s | C-4a, C-5, C-7, C-8 |
| 7 | 163.1 | 165.0 | 163.2 | ||||||
| 8 | 105.3 | 103.4 | 106.0 | ||||||
| 8a | 154.1 | 156.0 | 154.2 | ||||||
| 1′ | 131.5 | 131.5 | 131.5 | ||||||
| 2′,6′ | 126.5 | 7.91 m | C-2, C-4′, C-2′, C-6′ | 126.5 | 7.92 m | C-2, C-4′, C-2′, C-6′ | 126.4 | 7.93 m | C-2, C-4′, C-2′, C-6′ |
| 3′,5′ | 129.1 | 7.52 m | C-1′, C-3′, C-5′ | 129.4 | 7.59 m | C-1′, C-3′, C-5′ | 129.2 | 7.54 m | C-1′, C-3′, C-5′ |
| 4′ | 131.9 | 7.55 m | C-2′, C-6′ | 132.2 | 7.59 m | C-2′, C-6′ | 132.0 | 7.56 m | C-2′, C-6′ |
| 1″ | 114.9 | 6.85, d (16.5) | C-7, C-8a, C-2″, C-3″ | 132.0 | 8.06, d (16.4) | C-7, C-8a, C-2″, C-3″ | 117.5 | 6.83, d (16.5) | C-7, C-8a, C-2″, C-3″ |
| 2″ | 141.3 | 6.70, d (16.5) | C-8, C-3″, 3″-Me2 | 128.8 | 7.18, d (16.4) | C-8, C-3″, C-4″ | 135.4 | 6.29, d (16.5) | C-8, C-3″, C-4″, C-5″ |
| 3″ | 71.5 | 199.1 | 142.9 | ||||||
| 3″-Me2 | 30.0 | 1.50 s | C-2″, C-3″, 3″-Me2 | ||||||
| 4″ | 27.8 | 2.41 s | C-2″, C-3″ | 116.8 | 5.10 s | C-2″, C-3″, C-5″ | |||
| 5″ | 18.2 | 2.06 s | C-2″, C-3″, C-4″ | ||||||
| 7(OMe) | 56.1 | 3.92 s | C-7 | 56.4 | 4.01 s | C-7 | 56.2 | 3.97 s | C-7 |
| Position | δC | δH, m (J in Hz) | HMBC (H→C) |
|---|---|---|---|
| 2 | 160.6 | ||
| 3 | 110.1 | 6.55 s | C-2, C-4, C-4a, C-1′ |
| 4 | 177.2 | ||
| 4a | 109.1 | ||
| 5 | 162.9 | ||
| 6 | 91.1 | 6.41 s | C-4a, C-5, C-7, C-8 |
| 7 | 166.3 | ||
| 8 | 103.9 | ||
| 8a | 154.9 | ||
| 1′ | 131.7 | ||
| 2′,6′ | 126.3 | 7.70 m | C-2, C-4′, C-2′, C-6′ |
| 3′,5′ | 128.7 | 7.45 m | C-1′, C-3′, C-5′ |
| 4′ | 131.1 | 7.49 m | C-2′, C-6′ |
| 2″ | 83.9 | ||
| 3″ | 206.1 | ||
| 4″ | 47.7 | 4.95 dd (10.2, 6.1) | C-7, C-8, C-8a, C-2″, C-3″, C-5″ |
| 5″ | 75.8 | 4.90 dd (10.2, 8.8) | C-7, C-8, C-3″, C-4″ |
| 4.84 dd (6.1, 8.8) | C-7, C-8, C-3″, C-4″ | ||
| 2″-Me | 24.0 | 1.57 s | C-2″, C-3″, 2″-Me |
| 2″-Me | 23.9 | 1.65 s | C-2″, C-3″, 2″-Me |
| 5-OMe | 56.7 | 3.96 s | C-5 |
| 7-COMe | 170.0 | ||
| 7-COMe | 21.4 | 2.11 s | 7-COMe |
| Samples | Antiplasmodial Activity against P. falciparum | Cytotoxicity | |||
|---|---|---|---|---|---|
| D6 | LO2 * | BEAS * | A549 ** | HepG2 ** | |
| (E)-5-Hydroxytephrostachin (1) | 1.7 ± 0.1 | 21.7 ± 4.8 | 24.5 ± 2.7 | 76.1 ± 2.9 | >100 |
| Purleptone (2) | NT | >100 | >100 | >100 | >100 |
| Terpurlepflavone (4) | 14.8 ± 3.2 | >100 | >100 | >100 | >100 |
| Tachrosin (9) | 27.1 ± 3.2 | >100 | >100 | >100 | >100 |
| Chloroquine | 0.037 ± 0.003 | ||||
| Artesunate-Mefloquine | 0.075 ± 0.006 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atilaw, Y.; Muiva-Mutisya, L.; Ndakala, A.; Akala, H.M.; Yeda, R.; Wu, Y.J.; Coghi, P.; Wong, V.K.W.; Erdélyi, M.; Yenesew, A. Four Prenylflavone Derivatives with Antiplasmodial Activities from the Stem of Tephrosia purpurea subsp. leptostachya. Molecules 2017, 22, 1514. https://doi.org/10.3390/molecules22091514
Atilaw Y, Muiva-Mutisya L, Ndakala A, Akala HM, Yeda R, Wu YJ, Coghi P, Wong VKW, Erdélyi M, Yenesew A. Four Prenylflavone Derivatives with Antiplasmodial Activities from the Stem of Tephrosia purpurea subsp. leptostachya. Molecules. 2017; 22(9):1514. https://doi.org/10.3390/molecules22091514
Chicago/Turabian StyleAtilaw, Yoseph, Lois Muiva-Mutisya, Albert Ndakala, Hoseah M. Akala, Redemptah Yeda, Yu J. Wu, Paolo Coghi, Vincent K. W. Wong, Máté Erdélyi, and Abiy Yenesew. 2017. "Four Prenylflavone Derivatives with Antiplasmodial Activities from the Stem of Tephrosia purpurea subsp. leptostachya" Molecules 22, no. 9: 1514. https://doi.org/10.3390/molecules22091514
APA StyleAtilaw, Y., Muiva-Mutisya, L., Ndakala, A., Akala, H. M., Yeda, R., Wu, Y. J., Coghi, P., Wong, V. K. W., Erdélyi, M., & Yenesew, A. (2017). Four Prenylflavone Derivatives with Antiplasmodial Activities from the Stem of Tephrosia purpurea subsp. leptostachya. Molecules, 22(9), 1514. https://doi.org/10.3390/molecules22091514