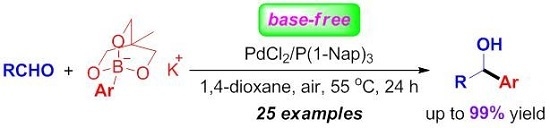
Efficient Approach to Carbinol Derivatives through Palladium-Catalyzed Base-Free Addition of Aryltriolborates to Aldehydes
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Carbinol Derivatives through the Palladium-Catalyzed Addition of Aryltriolborates to Aldehydes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmidt, F.; Stemmler, R.T.; Rudolph, J.; Bolm, C. Catalytic asymmetric approaches towards enantiomerically enriched diarylmethanols and diarylmethylamines. Chem. Soc. Rev. 2006, 35, 454–470. [Google Scholar] [PubMed]
- Seto, M.; Aramaki, Y.; Imoto, H.; Akikawa, K.; Oda, T.; Kanzaki, N.; Iizawa, Y.; Baba, M.; Shiraishi, M. Orally Active CCR5 Antagonists as Anti-HIV-1 Agents 2: Synthesis and Biological Activities of Anilide Derivatives Containing a Pyridine N-Oxide Moiety. Chem. Pharm. Bull. 2004, 52, 818–829. [Google Scholar] [CrossRef]
- Nilvebrant, L.; Andersson, K.-E.; Gillberg, P.-G.; Stahl, M.; Sparf, B. Tolterodine—A new bladder-selective antimuscarinic agent. Eur. J. Pharmacol. 1997, 327, 195–207. [Google Scholar] [CrossRef]
- Welch, W.M.; Kraska, A.R.; Sarges, R.; Coe, K.B. Nontricyclic antidepressant agents derived from cis- and trans-1-amino-4-aryltetralins. J. Med. Chem. 1984, 27, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Astles, P.C.; Brown, T.J.; Halley, F.; Handscombe, C.M.; Harris, N.V.; Majid, T.N.; McCarthy, C.; McLay, I.M.; Morley, A.; Porter, B.; et al. Selective ETA Antagonists. 5. Discovery and Structure-Activity Relationships of Phenoxyphenylacetic Acid Derivatives. J. Med. Chem. 2000, 43, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Hildebrand, J.P.; Muniz, K.; Hermanns, N. Catalyzed Asymmetric Arylation Reactions. Angew. Chem. Int. Ed. 2001, 40, 3284–3308. [Google Scholar] [CrossRef]
- Soai, K.; Kawase, Y. Enantioselective Furylation of Prochiral Aldehydes by Difurylrinc in the Presence of a Chiral Amino Alcohol: Asymmetric Synthesis of 2-Furylmethanols. J. Chem. Soc. Perkin Trans. 1 1990, 3214–3215. [Google Scholar] [CrossRef]
- Boymond, L.; Rottländer, M.; Cahiez, G.; Knochel, P. Preparation of Highly Functionalized Grignard Reagents by an Iodine-Magnesium Exchange Reaction and its Application in Solid-Phase Synthesis. Angew. Chem. Int. Ed. 1998, 37, 1701–1703. [Google Scholar] [CrossRef]
- Li, C.-J.; Meng, Y. Grignard-Type Carbonyl Phenylation in Water and under an Air Atmosphere. J. Am. Chem. Soc. 2000, 122, 9538–9539. [Google Scholar] [CrossRef]
- Wu, K.-H.; Gau, H.-M. Remarkably Efficient Enantioselective Titanium(IV)−(R)-H8-BINOLate Catalyst for Arylations to Aldehydes by Triaryl(tetrahydrofuran)aluminum Reagents. J. Am. Chem. Soc. 2006, 128, 14808–14809. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Rudolph, J. Catalyzed Asymmetric Aryl Transfer Reactions to Aldehydes with Boronic Acids as Aryl Source. J. Am. Chem. Soc. 2002, 124, 14850–14851. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Lüdtke, D.S.; Vargas, F.; Paixão, M.W. Catalytic enantioselective arylation of aldehydes: Boronic acids as a suitable source of transferable aryl groups. Chem. Commun. 2005, 2512–2514. [Google Scholar] [CrossRef] [PubMed]
- Infante, R.; Nieto, J.; Andrés, C. Asymmetric additive-free aryl addition to aldehydes using perhydrobenzoxazines as ligands and boroxins as aryl source. Org. Biomol. Chem. 2011, 9, 6691–6699. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Organoborates in New Synthetic Reactions. Acc. Chem. Res. 1982, 15, 178–184. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1998, 576, 147–168. [Google Scholar] [CrossRef]
- Sakai, M.; Ueda, M.; Miyaura, N. Rhodium-Catalyzed Addition of Organoboronic Acids to Aldehydes. Angew. Chem. Int. Ed. 1998, 37, 3279–3281. [Google Scholar] [CrossRef]
- Gois, P.M.P.; Trindade, A.F.; Veiros, L.F.; André, V.; Duarte, M.T.; Afonso, C.A.M.; Caddick, S.; Cloke, F.G.N. Tuning the Reactivity of Dirhodium(II) Complexes with Axial N-Heterocyclic Carbene Ligands: The Arylation of Aldehydes. Angew. Chem. Int. Ed. 2007, 46, 5750–5753. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Peng, Y.; Zhang, J.; Lu, H.; Feng, X.; Ge, R.; Dong, B.; Wang, B.; Hu, X.; Gao, Y. Sophisticated Design of Covalent Organic Frameworks with Controllable Bimetallic Docking for a Cascade Reaction. Chem. Eur. J. 2016, 22, 9087–9091. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cai, C. A simple procedure for the polymer-supported N-heterocyclic carbene–rhodium complex via click chemistry: A recyclable catalyst for the addition of arylboronic acids to aldehydes. Chem. Commun. 2011, 47, 12319–12321. [Google Scholar] [CrossRef] [PubMed]
- Desroches, J.; Tremblay, A.; Paquin, J.-F. Racemic and enantioselective metal-catalyzed synthesis of SF5-containing diarylmethanols. Org. Biomol. Chem. 2016, 14, 8764–8780. [Google Scholar] [CrossRef] [PubMed]
- Trindade, A.F.; Gois, P.M.P.; Veiros, L.F.; André, V.; Duarte, M.T.; Afonso, C.A.M.; Caddick, S.; Geoffrey, F.; Cloke, N. Axial Coordination of NHC Ligands on Dirhodium(II) Complexes: Generation of a New Family of Catalysts. J. Org. Chem. 2008, 73, 4076–4086. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, X.; Wang, Z. The carbonyl group tuned electron-deficient phosphorus ligands and their application in Rhodium catalyzed arylation to aldehydes. Tetrahedron Lett. 2015, 56, 5673–5675. [Google Scholar] [CrossRef]
- Tan, J.; Kuang, Y.; Wang, Y.; Huang, Q.; Zhu, J.; Wang, Y. Axial Tri-tert-butylphosphane Coordination to Rh2(OAc)4: Synthesis, Structure, and Catalytic Studies. Organometallics 2016, 35, 3139–3147. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ohta, T.; Ito, Y. Palladium-Catalyzed Addition of Arylboronic Acids to Aldehydes. Org. Lett. 2005, 7, 4153–4155. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Lu, Y.; Dong, C.; Hu, Q. Anionic Four-Electron Donor-Based Palladacycles as Catalysts for Addition Reactions of Arylboronic Acids with α,β-Unsaturated Ketones, Aldehydes, and α-Ketoesters. Org. Lett. 2007, 9, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, M.; Shimazawa, R.; Shirai, R. Efficient 1,2-Addition of Aryl- and Alkenylboronic Acids to Aldehydes Catalyzed by the Palladium/Thioether−Imidazolinium Chloride System. J. Org. Chem. 2008, 73, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xing, C.; Israeland, M.; Hu, Q. A nontransmetalation reaction pathway for anionic four-electron donor-based palladacycle-catalyzed addition reactions of arylborons with aldehydes. Tetrahedron Lett. 2011, 52, 3324–3328. [Google Scholar] [CrossRef]
- Zou, T.; Pi, S.; Li, J. FeCl3-Catalyzed 1,2-Addition Reactions of Aryl Aldehydes with Arylboronic Acids. Org. Lett. 2009, 11, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, J.; Jeganmohan, M.; Cheng, C. Cobalt-Catalyzed Addition Reaction of Organoboronic Acids with Aldehydes: Highly Enantioselective Synthesis of Diarylmethanols. Chem. Eur. J. 2010, 16, 8989–8992. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, G.; Shirakawa, E.; Tsuchimoto, T.; Kawakami, Y. Alkynes as activators in the nickel-catalysed addition of organoboronates to aldehydes. Chem. Commun. 2005, 21, 1459–1461. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Kondo, K.; Aoyama, T. Et-duphos-nickel-catalyzed asymmetric arylation of benzaldehyde derivatives bearing an ortho-Me2PhSi group with potassium aryltriolborates. Tetrahedron Lett. 2009, 50, 6001–6003. [Google Scholar] [CrossRef]
- Qin, C.; Chen, J.; Wu, H.; Cheng, J.; Zhang, Q.; Zuo, B.; Su, W.; Ding, J. One-pot synthesis of diaryl ketones from aldehydes via palladium-catalyzed reaction with aryl boronic acids. Tetrahedron Lett. 2008, 49, 1884–1888. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, J.; Chen, J.; Liu, M.; Gao, W.; Wu, H. Copper-Catalyzed Arylation of Arylboronic Acids with Aldehydes. Synlett 2011, 2011, 1626–1630. [Google Scholar]
- Zheng, H.; Zhang, Q.; Chen, J.; Liu, M.; Cheng, S.; Ding, J.; Wu, H.; Su, W. Copper(II) Acetate-Catalyzed Addition of Arylboronic Acids to Aromatic Aldehydes. J. Org. Chem. 2009, 74, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wu, H.; Cheng, J.; Chen, X.; Liu, M.; Zhang, W.; Su, W.; Ding, J. The Palladium-Catalyzed Addition of Aryl- and Heteroarylboronic Acids to Aldehydes. J. Org. Chem. 2007, 72, 4102–4107. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wu, H.; Chen, J.; Liu, M.; Cheng, J.; Su, W.; Ding, J. Palladium-Catalyzed Aromatic Esterification of Aldehydes with Organoboronic Acids and Molecular Oxygen. Org. Lett. 2008, 10, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y. Cyclic Triolborate Salts: Novel Reagent for Organic Synthesis. Heterocycles 2012, 85, 799–819. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Takizawa, M.; Yu, X.; Miyaura, N. Cyclic Triolborates: Air- and Water-Stable Ate Complexes of Organoboronic Acids. Angew. Chem. Int. Ed. 2008, 47, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhao, S.; Xu, N. Aryltriolborates as Air- and Water-Stable Bases for Wittig Olefination. Synthesis 2015, 47, 359–366. [Google Scholar] [CrossRef]
- Yu, X.; Yamamoto, Y.; Miyaura, N. Aryl Triolborates: Novel Reagent for Copper-Catalyzed N Arylation of Amines, Anilines, and Imidazoles. Chem. Asian J. 2008, 3, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Ikizakura, K.; Ito, H.; Miyaura, N. Cross-Coupling Reaction with Lithium Methyltriolborate. Molecules 2013, 18, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, S.; Takizawa, M.; Sugai, J.; Ito, H.; Yamamoto, Y. Tetrabutylammonium 2-Pyridyltriolborate Salts for Suzuki-Miyaura Cross-Coupling Reactions with Aryl Chlorides. Org. Lett. 2013, 15, 4308–4311. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qiao, R.; Chen, J.; Huang, X.; Liu, M.; Gao, W.; Ding, J.; Wu, H. Palladium-Catalyzed Cascade Reaction of 2-Amino-N′-arylbenzohydrazides with Triethyl Orthobenzoates to Construct Indazolo[3,2-b]quinazolinones. J. Org. Chem. 2015, 80, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Wu, G.; Liu, M.; Gao, W.; Ding, J.; Chen, J.; Huang, X.; Wu, H. Copper-Catalyzed Oxirane-Opening Reaction with Aryl Iodides and Se Powder. J. Org. Chem. 2016, 81, 7584–7590. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, J.; Huang, X.; Ding, J.; Liu, M.; Wu, H. Pd-Catalyzed Intramolecular Aerobic Oxidative C–H Amination of 2-Aryl-3-(arylamino)quinazolinones: Synthesis of Fluorescent Indazolo[3,2-b]quinazolinones. Org. Lett. 2014, 16, 5418–5421. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, L.; Su, W. Palladium-catalyzed direct addition of arylboronic acids to 2-aminobenzonitrile derivatives: Synthesis, biological evaluation and in silico analysis of 2-aminobenzophenones, 7-benzoyl-2-oxoindolines, and 7-benzoylindoles. Org. Biomol. Chem. 2014, 12, 8204–8211. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Xu, L.; Wang, Q.; Chen, J.; Ding, J.; Wu, H. Palladium-Catalyzed Addition of Potassium Aryltrifluoroborates to Aliphatic Nitriles: Synthesis of Alkyl Aryl Ketones, Diketone Compounds, and 2-Arylbenzo[b]furans. J. Org. Chem. 2013, 78, 5273–5281. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, Y.; Liu, M.; Ding, J.; Su, W.; Wu, H. Palladium-Catalyzed Aerobic Oxidative Coupling of Acyl Chlorides with Arylboronic Acids. Adv. Synth. Catal. 2012, 354, 2117–2122. [Google Scholar] [CrossRef]
- Lu, W.; Chen, J.; Liu, M.; Ding, J.; Gao, W.; Wu, H. Palladium-Catalyzed Decarboxylative Coupling of Isatoic Anhydrides with Arylboronic Acids. Org. Lett. 2011, 13, 6114–6117. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Hu, K.; Yu, S.; Zhu, J.; Cheng, T.; Wang, X.; Chen, J.; Wu, H. Tandem Addition/Cyclization for Access to Isoquinolines and Isoquinolones via Catalytic Carbopalladation of Nitriles. Org. Lett. 2017, 19, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Qi, L.; Yu, S.; Cheng, T.; Wang, X.; Li, Z.; Xia, Y.; Chen, J.; Wu, H. Efficient synthesis of isoquinolines in water by a alkylnitriles with arylboronic acids. Green Chem. 2017, 19, 1740–1750. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




| Entry | Pd Source | Pd/L Ratio (x) | Solvent | Yield b (%) |
|---|---|---|---|---|
| 1 c | PdCl2 | 1 | THF | trace |
| 2 | PdCl2 | 1 | THF | 18 |
| 3 | PdCl2 | 1 | Toluene | 43 |
| 4 | PdCl2 | 1 | xylene | 42 |
| 5 | PdCl2 | 1 | n-hexane | 71 |
| 6 | PdCl2 | 1 | ethyl acetate | 38 |
| 7 | PdCl2 | 1 | isopropanol | trace |
| 8 | PdCl2 | 1 | 1,4-dioxane | 87(30) e (57) f |
| 9 | PdCl2 | 1 | DMF | trace |
| 10 | Pd(OAc)2 | 1 | 1,4-dioxane | 49 |
| 11 | Pd(TFA)2 | 1 | 1,4-dioxane | 48 |
| 12 | PdCl2(PPh3)2 | 1 | 1,4-dioxane | 23 |
| 13 | PdCl2(dppf) | 1 | 1,4-dioxane | 61 |
| 14 | PdCl2(dppe) | 1 | 1,4-dioxane | 41 |
| 15 | PdCl2(cod) | 1 | 1,4-dioxane | 43 |
| 16 | PdCl2(Py)2 | 1 | 1,4-dioxane | 10 |
| 17 | PdCl2(MeCN)2 | 1 | 1,4-dioxane | 29 |
| 18 | Pd(PPh3)4 | 1 | 1,4-dioxane | 38 |
| 19 | Pd2(dba)3 | 1 | 1,4-dioxane | 53 |
| 20 | PdCl2 | 0.5 | 1,4-dioxane | 68 |
| 21 | PdCl2 | 1.5 | 1,4-dioxane | 75 |
| 22 | PdCl2 | 2 | 1,4-dioxane | 79 |
| 23 | PdCl2 | 1 | 1,4-dioxane | 85 d |

 |  |  |  |  |
| 3a (94%) | 3b (87%) | 3c (84%) | 3d (96%) | 3e (99%) |
 |  |  |  |  |
| 3f (94%) | 3g (95%) | 3h (72%) | 3i (95%) | 3j (91%) |
 |  |  |  |  |
| 3k (83%) | 3l (83%) | 3m (86%) | 3n (87%) | 3o (84%) |
 |  | |||
| 1p (0%) | 1q (0%) |

 |  |  |  |
| 3a (94%) | 3p (90%) | 3q (95%) | 3r (91%) |
 |  |  |  |
| 3d (96%) | 3s (91%) | 3t (99%) | 3u (96%) |
 |  |  |  |
| 3v (97%) | 3w (93%) | 3x (89%) | 3y (85%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.; Ye, P.; Zhen, Q.; Yao, X.; Xu, T.; Shao, Y.; Chen, J. Efficient Approach to Carbinol Derivatives through Palladium-Catalyzed Base-Free Addition of Aryltriolborates to Aldehydes. Molecules 2017, 22, 1580. https://doi.org/10.3390/molecules22091580
Hu K, Ye P, Zhen Q, Yao X, Xu T, Shao Y, Chen J. Efficient Approach to Carbinol Derivatives through Palladium-Catalyzed Base-Free Addition of Aryltriolborates to Aldehydes. Molecules. 2017; 22(9):1580. https://doi.org/10.3390/molecules22091580
Chicago/Turabian StyleHu, Kun, Pengqing Ye, Qianqian Zhen, Xinrong Yao, Tong Xu, Yinlin Shao, and Jiuxi Chen. 2017. "Efficient Approach to Carbinol Derivatives through Palladium-Catalyzed Base-Free Addition of Aryltriolborates to Aldehydes" Molecules 22, no. 9: 1580. https://doi.org/10.3390/molecules22091580
APA StyleHu, K., Ye, P., Zhen, Q., Yao, X., Xu, T., Shao, Y., & Chen, J. (2017). Efficient Approach to Carbinol Derivatives through Palladium-Catalyzed Base-Free Addition of Aryltriolborates to Aldehydes. Molecules, 22(9), 1580. https://doi.org/10.3390/molecules22091580