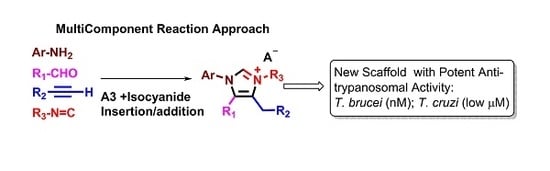
Tetrasubstituted Imidazolium Salts as Potent Antiparasitic Agents against African and American Trypanosomiases
Abstract
:1. Introduction
2. Results and Discussion
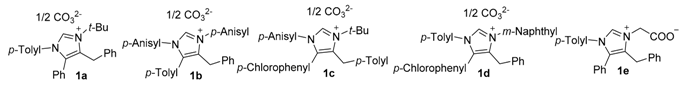
2.1. Chemistry
2.2. Biological Activity against T. brucei and T. cruzi
2.3. Molecular Properties of Compounds 1
3. Materials and Methods
3.1. Compound Preparation General Methods
3.2. Biological Activity Determination
3.2.1. T. brucei Culturing and Evaluation of Trypanocidal Activity
3.2.2. T. cruzi Culturing and Evaluation of Trypanocidal Activity
3.2.3. Cytotoxic Activity against Rat Skeletal Myoblast L6 Cells
3.3. Cheminformatics Calculations
3.4. Determination of Brain Permeability: PAMPA-BBB Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Word Health Organization Media Centre. Trypanosomiasis, Human African (Sleeping Sickness). Available online: http://www.who.int/mediacentre/factsheets/fs259/en/ (accessed on 15 November 2017).
- Word Health Organization Media Centre. Chagas Disease (American Trypanosomiasis). Available online: http://www.who.int/mediacentre/factsheets/fs340/en/ (accessed on 15 November 2017).
- Njoroge, M.; Njuguna, N.M.; Mutai, P.; Ongarora, D.S.B.; Smith, P.W.; Chibale, K. Recent approaches to chemical discovery and development against malaria and the neglected tropical diseases human African trypanosomiasis and schistosomiasis. Chem. Rev. 2014, 114, 11138–11163. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L. Multi-target-directed ligands as innovative tools to combat trypanosomatid diseases. Curr. Top. Med. Chem. 2011, 11, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Wittlin, S.; Rottmann, M.; Wenzler, T.; Kaiser, M.; Brun, R. Antiparasitic agents: New drugs on the horizon. Curr. Opin. Pharmacol. 2012, 12, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C.; Horn, D.; Fairlamb, A.H.; Ferguson, M.A.; Gray, D.W.; Read, K.D.; De Rycker, M.; Torrie, L.S.; Wyatt, P.G.; Wyllie, S.; et al. Anti-trypanosomatid drug discovery: An ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017, 15, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Neglected Tropical Diseases. Available online: http://www.cdc.gov/globalhealth/ntd/ (accessed on 20 November 2017).
- Gravel, J.; Schmitzer, A.R. Imidazolium and benzimidazolium-containing compounds: From simple toxic salts to highly bioactive drugs. Org. Biomol. Chem. 2017, 15, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Faral-Tello, P.; Liang, M.; Mahler, G.; Wipf, P.; Robello, C. Imidazolium compounds are active against all stages of Trypanosoma cruzi. Int. J. Antimicrob. Agents 2014, 43, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kishore, K.G.; Ghashghaei, O.; Estarellas, C.; Mestre, M.M.; Monturiol, C.; Kielland, N.; Kelly, J.M.; Francisco, A.F.; Jayawardhana, S.; Muñoz-Torrero, D.; et al. Insertion of isocyanides into N–Si bonds: Multicomponent reactions with azines leading to potent antiparasitic compounds. Angew. Chem. Int. Ed. 2016, 55, 8994–8998. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaei, O.; Revés, M.; Kielland, N.; Lavilla, R. Modular access to tetrasubstituted imidazolium salts through acid-catalyzed addition of isocyanides to propargylamines. Eur. J. Org. Chem. 2015, 4383–4388. [Google Scholar] [CrossRef]
- Li, C.-J.; Wei, C. Highly efficient Grignard-type imine additions via C–H activation in water and under solvent-free conditions. Chem. Commun. 2002, 268–269. [Google Scholar] [CrossRef]
- Tong, S.; Wang, Q.; Wang, M.-X.; Zhu, J. Tuning the reactivity of isocyano group: Synthesis of imidazoles and imidazoliums from propargylamines and isonitriles in the presence of multiple catalysts. Angew. Chem. Int. Ed. 2015, 54, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 4–25. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Viswanadhan, V.N.; Balan, C.; Hulme, C.; Cheetham, J.C.; Sun, Y. Knowledge-based approaches in the design and selection of compound libraries for drug discovery. Curr. Opin. Drug Discov. Dev. 2002, 5, 400–406. [Google Scholar] [PubMed]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Moving beyond rules: The development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem. Neurosci. 2010, 1, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Central nervous system multiparameter optimization desirability: Application in drug discovery. ACS Chem. Neurosci. 2016, 7, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Calculations were Performed Using the Molinspiration Package. Available online: http://molinspiration.com (accessed on 11 December 2017).
- Wilkinson, S.R.; Prathalingam, S.R.; Taylor, M.C.; Ahmed, A.; Horn, D.; Kelly, J.M. Functional characterisation of the iron superoxide dismutase gene repertoire in Trypanosoma brucei. Free Radic. Biol. Med. 2006, 40, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Kendall, G.; Wilderspin, A.F.; Ashall, F.; Miles, M.A.; Kelly, J.M. Trypanosoma cruzi glycosomal glyceraldehyde-3-phosphate dehydrogenase does not conform to the “hotspot” topogenic signal model. EMBO J. 1990, 9, 2751–2758. [Google Scholar] [PubMed]
- Bot, C.; Hall, B.S.; Bashir, N.; Taylor, M.C.; Helsby, N.A.; Wilkinson, S.R. Trypanocidal activity of aziridinyl nitrobenzamide prodrugs. Antimicrob. Agents Chemother. 2010, 54, 4246–4252. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a–e are not available from the authors. |


| Compd. | R1 | R2 | R3 | R4 |  |
| 1a | p-Me-Ph | Ph | Ph | tert-Bu | |
| 1b | p-MeO-Ph | p-Me-Ph | Ph | p-MeO-Ph | |
| 1c | p-MeO-Ph | p-Cl-Ph | p-Me-Ph | tert-Bu | |
| 1d | p-Me-Ph | p-Cl-Ph | Ph | 2-naphthyl | |
| 1e | p-Me-Ph | Ph | Ph | -COOH |
| 1a | 1b | 1c | 1d | 1e | |
|---|---|---|---|---|---|
| EC50 1 (T. brucei, µM) | 0.18 ± 0. 01 | 0.04 ± 0.00 | 0.18 ± 0.01 | 0.07 ± 0.00 | 5.26 ± 0.01 |
| EC90 2 (T. brucei, µM) | 0.28 ± 0.07 | 0.05 ± 0.00 | 0.20 ± 0.01 | 0.09 ± 0.00 | 6.79 ± 0.09 |
| EC50 (L6 cells, µM) | 33.90 ± 3.30 | 4.60 ± 0.38 | 9.69 ± 0.58 | 4.09 ± 0.28 | >130 |
| SI 3 (T. brucei) | 188 | 118 | 54 | 56 | >24 |
| EC50 (T. cruzi, µM) | 1.85 ± 0.29 | 0.44 ± 0.01 | 1.72 ± 0.39 | 0.54 ± 0.08 | >20 |
| EC90 (T. cruzi, µM) | 3.41 ± 0.14 | 1.29 ± 0.05 | 2.98 ± 0.19 | 1.00 ± 0.04 | >20 |
| SI (T. cruzi) | 18 | 10 | 5.6 | 7.6 | - |
| 1a | 1b | 1c | 1d | 1e | |
|---|---|---|---|---|---|
| PAMPA-BBB (Pe) | 4.2 | 4.9 | 2.6 | 2.4 | 1.6 |
| CNS MPO | 3.3 | 3.5 | 3.7 | 1.4 | 5.8 |
| miLogP | 3.30 | 3.93 | 4.04 | 5.67 | −1.35 |
| MW | 381.54 | 461.58 | 446.01 | 486.04 | 382.46 |
| nOHNH | 0 | 0 | 0 | 0 | 0 |
| nON | 2 | 4 | 3 | 2 | 4 |
| nviolations | 0 | 0 | 0 | 1 | 0 |
| TPSA | 8.82 | 27.29 | 18.05 | 8.82 | 48.95 |
| nrotb | 5 | 7 | 6 | 5 | 6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghashghaei, O.; Kielland, N.; Revés, M.; Taylor, M.C.; Kelly, J.M.; Di Pietro, O.; Muñoz-Torrero, D.; Pérez, B.; Lavilla, R. Tetrasubstituted Imidazolium Salts as Potent Antiparasitic Agents against African and American Trypanosomiases. Molecules 2018, 23, 177. https://doi.org/10.3390/molecules23010177
Ghashghaei O, Kielland N, Revés M, Taylor MC, Kelly JM, Di Pietro O, Muñoz-Torrero D, Pérez B, Lavilla R. Tetrasubstituted Imidazolium Salts as Potent Antiparasitic Agents against African and American Trypanosomiases. Molecules. 2018; 23(1):177. https://doi.org/10.3390/molecules23010177
Chicago/Turabian StyleGhashghaei, Ouldouz, Nicola Kielland, Marc Revés, Martin C. Taylor, John M. Kelly, Ornella Di Pietro, Diego Muñoz-Torrero, Belén Pérez, and Rodolfo Lavilla. 2018. "Tetrasubstituted Imidazolium Salts as Potent Antiparasitic Agents against African and American Trypanosomiases" Molecules 23, no. 1: 177. https://doi.org/10.3390/molecules23010177
APA StyleGhashghaei, O., Kielland, N., Revés, M., Taylor, M. C., Kelly, J. M., Di Pietro, O., Muñoz-Torrero, D., Pérez, B., & Lavilla, R. (2018). Tetrasubstituted Imidazolium Salts as Potent Antiparasitic Agents against African and American Trypanosomiases. Molecules, 23(1), 177. https://doi.org/10.3390/molecules23010177