Molecularly Imprinted Microrods via Mesophase Polymerization
Abstract
:1. Introduction
2. Results and Discussion
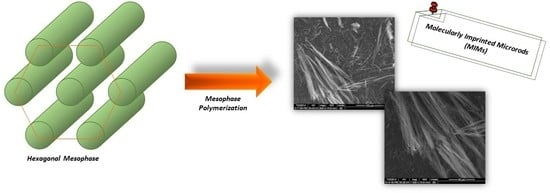
2.1. Synthesis of Molecularly Imprinted Microrods (MIMs) via Mesophase Polymerization
2.2. Imprinting Effect and Selectivity Assessment
2.3. In Vitro Drug Release Studies
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of Molecularly Imprinted Microrods (MIMs) via Mesophase Polymerization
3.4. Binding Studies: Imprinting Effect and Selectivity Assessment
3.5. Drug Loading by Soaking Procedure and In Vitro Release Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Doroudian, G.; Pinney, J.; Ayala, P.; Los, T.; Desai, T.A.; Russell, B. Sustained delivery of MGF peptide from microrods attracts stem cells and reduces apoptosis of myocytes. Biomed. Microdevices 2014, 16, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Luliński, P. Molecularly imprinted polymers based drug delivery devices: A way to application in modern pharmacotherapy. A review. Mater. Sci. Eng. C 2017, 76, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Fareghi, A.R.; Moghadam, P.N.; Khalafy, J.; Bahram, M.; Moghtader, M. Preparation of a new molecularly imprinted polymer based on self-crosslinkable cellulose acrylate in aqueous solution: A drug delivery system for furosemide. J. Appl. Polym. Sci. 2017, 134, 45581. [Google Scholar] [CrossRef]
- Parisi, O.I.; Morelli, C.; Puoci, F.; Saturnino, C.; Caruso, A.; Sisci, D.; Trombino, G.E.; Picci, N.; Sinicropi, M.S. Magnetic molecularly imprinted polymers (MMIPs) for carbazole derivative release in targeted cancer therapy. J. Mater. Chem. B 2014, 2, 6619–6625. [Google Scholar] [CrossRef]
- Puoci, F.; Cirillo, G.; Curcio, M.; Iemma, F.; Parisi, O.I.; Castiglione, M.; Picci, N. Molecularly imprinted polymers for α-tocopherol delivery. Drug Deliv. 2008, 15, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H.; Guo, H.; Wang, Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018, 100, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Menaker, A.; Syritski, V.; Reut, J.; Öpik, A.; Horváth, V.; Gyurcsányi, R.E. Electrosynthesized Surface-Imprinted Conducting Polymer Microrods for Selective Protein Recognition. Adv. Mater. 2009, 21, 2271–2275. [Google Scholar] [CrossRef]
- Ceolin, G.; Orbán, Á.; Kocsis, V.; Gyurcsányi, R.E.; Kézsmárki, I.; Horváth, V. Electrochemical template synthesis of protein-imprinted magnetic polymer microrods. J. Mater. Sci. 2013, 48, 5209–5218. [Google Scholar] [CrossRef]
- Suriyanarayanan, S.; Nawaz, H.; Ndizeye, N.; Nicholls, I.A. Hierarchical thin film architectures for enhanced sensor performance: liquid crystal-mediated electrochemical synthesis of nanostructured imprinted polymer films for the selective recognition of bupivacaine. Biosensors 2014, 4, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.O.; Oluwafemi, O.S.; Songca, S.P.; Sukhbaatar, A.; Mori, S.; Okajima, J.; Komiya, A.; Maruyama, S.; Kodama, T. A novel treatment for metastatic lymph nodes using lymphatic delivery and photothermal therapy. Sci. Rep. 2017, 7, 45459. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jain, N.; Sareen, R. Nanocarriers for diagnosis and targeting of breast cancer. BioMed Res. Int. 2013, 2013, 960821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ma, X.; Jin, E.; Tang, J.; Sui, M.; Shen, Y.; Van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials 2013, 34, 5722–5735. [Google Scholar] [CrossRef] [PubMed]
- Biazar, E. Application of polymeric nanofibers in medical designs, part III: Musculoskeletal and urological tissues. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 28–37. [Google Scholar] [CrossRef]
- James, H.P.; John, R.; Alex, A.; Anoop, K. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Girones Molera, J.; Alberto Mendez, J.; San Roman, J. Bioresorbable and Nonresorbable Polymers for Bone Tissue Engineering Jordi Girones. Curr. Pharm. Des. 2012, 18, 2536–2557. [Google Scholar] [CrossRef]
- Traitel, T.; Goldbart, R.; Kost, J. Smart polymers for responsive drug-delivery systems. J. Biomater. Sci. Polym. Ed. 2008, 19, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Mezzenga, R. Physics of Self-Assembly of Lyotropic Liquid Crystals. In Self-Assembled Supramolecular Architectures: Lyotropic Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–20. [Google Scholar]
- DePierro, M.A.; Guymon, C.A. Polymer structure development in lyotropic liquid crystalline solutions. Macromolecules 2014, 47, 5728–5738. [Google Scholar] [CrossRef]
- Yan, F.; Texter, J. Polymerization of and in mesophases. Adv. Colloid Interface Sci. 2006, 128, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Sikiti, P.; Msagati, T.A.; Mamba, B.B.; Mishra, A.K. Synthesis and characterization of molecularly imprinted polymers for the remediation of PCBs and dioxins in aqueous environments. J. Environ. Health Sci. Eng. 2014, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yun, Y.; Zhang, W.; Wang, L. Preparation, recognition characteristics and properties for quercetin molecularly imprinted polymers. Desalination Water Treat. 2011, 34, 309–314. [Google Scholar] [CrossRef]
- Cywinski, P. Fluorescent Molecularly Imprinted Polymers in Sensing of cAMP and cGMP. J. Phys. Chem. Biophys. 2013, 3. [Google Scholar] [CrossRef]
- Parisi, O.I.; Cirillo, G.; Curcio, M.; Puoci, F.; Iemma, F.; Spizzirri, U.G.; Picci, N. Surface modifications of molecularly imprinted polymers for improved template recognition in water media. J. Polym. Res. 2010, 17, 355–362. [Google Scholar] [CrossRef]
- Yildiz, T.; Kazanci, N. Investigation of temperature dependence of mesomorphism and refracting index of sodium dodecylsulphate+ water+ decanol lyotropic system. J. Mol. Struct. 2008, 886, 158–165. [Google Scholar] [CrossRef]


| Incubation Time (h) | Bound THEO (%) 0.6 mM | Bound THEO (%) 0.06 mM | ||
|---|---|---|---|---|
| MIMs | NIMs | MIMs | NIMs | |
| 3 | 45.8 ± 1.1 | 58.4 ± 0.8 | 23.4 ± 1.0 | 33.2 ± 1.1 |
| 6 | 50.6 ± 0.9 | 42.5 ± 0.7 | 42.7 ± 0.6 | 31.5 ± 0.7 |
| 24 | 60.7 ± 0.8 | 46.6 ± 0.7 | 49.2 ± 0.7 | 39.1 ± 1.2 |
| Imprinting Efficiency (α) | Selectivity Coefficient (ε) | |
|---|---|---|
| αTHEO | αCAFF | |
| 1.3 | 1.0 | 1.5 |
| DLC (%) | DLE (%) | ||
|---|---|---|---|
| MIMs | NIMs | MIMs | NIMs |
| 8.9 ± 0.6 | 7.7 ± 0.8 | 89.0 ± 0.7 | 76.5 ± 0.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, O.I.; Scrivano, L.; Candamano, S.; Ruffo, M.; Vattimo, A.F.; Spanedda, M.V.; Puoci, F. Molecularly Imprinted Microrods via Mesophase Polymerization. Molecules 2018, 23, 63. https://doi.org/10.3390/molecules23010063
Parisi OI, Scrivano L, Candamano S, Ruffo M, Vattimo AF, Spanedda MV, Puoci F. Molecularly Imprinted Microrods via Mesophase Polymerization. Molecules. 2018; 23(1):63. https://doi.org/10.3390/molecules23010063
Chicago/Turabian StyleParisi, Ortensia Ilaria, Luca Scrivano, Sebastiano Candamano, Mariarosa Ruffo, Anna Francesca Vattimo, Maria Vittoria Spanedda, and Francesco Puoci. 2018. "Molecularly Imprinted Microrods via Mesophase Polymerization" Molecules 23, no. 1: 63. https://doi.org/10.3390/molecules23010063
APA StyleParisi, O. I., Scrivano, L., Candamano, S., Ruffo, M., Vattimo, A. F., Spanedda, M. V., & Puoci, F. (2018). Molecularly Imprinted Microrods via Mesophase Polymerization. Molecules, 23(1), 63. https://doi.org/10.3390/molecules23010063